Global Photopheresis Products Market
Market Size in USD Million
CAGR :
% 
 USD
364.24 Million
USD
541.84 Million
2025
2033
USD
364.24 Million
USD
541.84 Million
2025
2033
| 2026 –2033 | |
| USD 364.24 Million | |
| USD 541.84 Million | |
|
|
|
|
Photopheresis Products Market Size
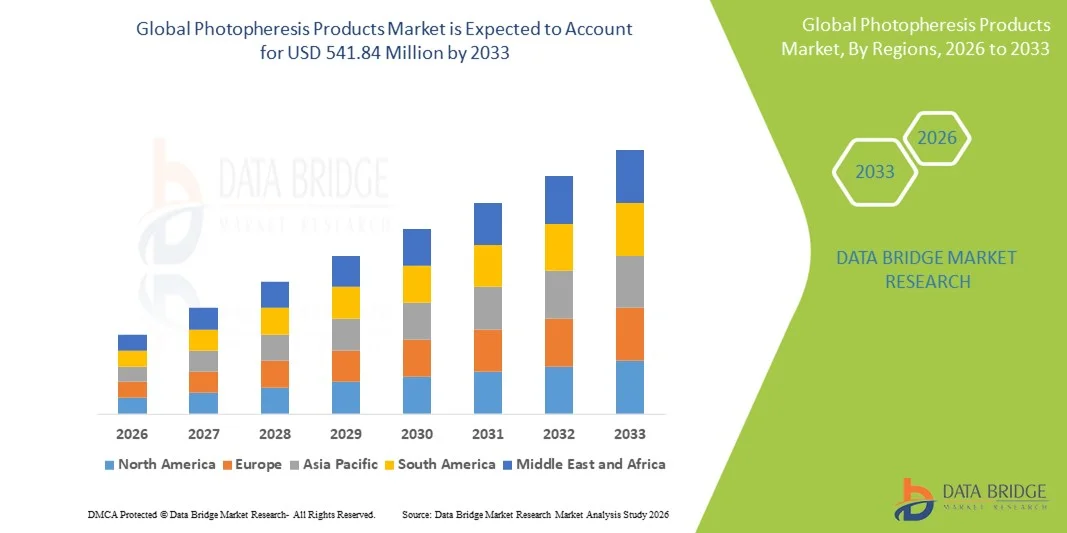
- The global photopheresis products market size was valued at USD 364.24 Million in 2025 and is expected to reach USD 541.84 Million by 2033, at a CAGR of 5.09% during the forecast period
- The market growth is largely fueled by the growing adoption and technological advancements in extracorporeal blood treatment technologies, leading to increased use of photopheresis products in hospital and specialty care settings for immune-mediated and hematological condition
- Furthermore, rising demand from healthcare providers for safe, effective, and minimally invasive immunomodulatory therapies is establishing photopheresis products as a preferred treatment option for conditions such as graft-versus-host disease, cutaneous T-cell lymphoma, and transplant rejection. These converging factors are accelerating the uptake of Photopheresis Products solutions, thereby significantly boosting the industry's growth
Photopheresis Products Market Analysis
- Photopheresis products, which include photopheresis systems, disposables, and related consumables used in extracorporeal photopheresis therapy, are increasingly vital in modern clinical practice due to their effectiveness in treating immune-mediated and hematological disorders in hospital and specialty care settings
- The escalating demand for photopheresis products is primarily driven by the rising incidence of conditions such as graft-versus-host disease (GvHD), cutaneous T-cell lymphoma (CTCL), and organ transplant rejection, along with growing adoption of immunomodulatory therapies that offer targeted treatment with favorable safety
- North America dominated the photopheresis products market with the largest revenue share of 41.8% in 2025, supported by advanced healthcare infrastructure, high adoption of specialty therapies, strong presence of leading manufacturers, and widespread clinical use of photopheresis in oncology and transplant centers, with the U.S. accounting for a significant portion of regional demand
- Asia-Pacific is expected to be the fastest-growing region in the photopheresis products market during the forecast period, driven by increasing awareness of advanced immunotherapies, expanding healthcare infrastructure, rising prevalence of autoimmune and hematological disorders, and growing investments in specialty treatment facilities across emerging economies
- The closed system segment dominated the largest market revenue share of 64.8% in 2025, driven by its superior safety profile, reduced risk of contamination, and higher level of automation
Report Scope and Photopheresis Products Market Segmentation
|
Attributes |
Photopheresis Products Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Photopheresis Products Market Trends
Advancement of Therapeutic Apheresis Technologies and Expanded Clinical Applications
- A significant and accelerating trend in the global photopheresis products market is the advancement of therapeutic apheresis technologies and the expanding use of photopheresis in the treatment of immune-mediated and hematological disorders
- For instance, extracorporeal photopheresis (ECP) is increasingly being adopted for conditions such as graft-versus-host disease (GVHD), cutaneous T-cell lymphoma (CTCL), and organ transplant rejection, supported by growing clinical evidence and guideline recommendations
- Continuous improvements in photopheresis systems, including enhanced cell separation efficiency, optimized ultraviolet light exposure, and closed-system designs, are improving treatment safety, consistency, and clinical outcomes
- The growing availability of disposable kits and standardized consumables is also supporting broader adoption by reducing cross-contamination risks and simplifying procedural workflows in hospitals and specialty clinics
- In addition, increasing focus on personalized and immunomodulatory therapies is strengthening the role of photopheresis as a targeted treatment option with fewer systemic side effects compared to conventional immunosuppressive therapies
- This trend toward safer, more efficient, and clinically validated photopheresis solutions is reshaping treatment approaches for complex immune disorders and supporting long-term market growth globally
Photopheresis Products Market Dynamics
Driver
Rising Prevalence of Immune-Related Disorders and Transplant Procedures
- The growing incidence of immune-mediated diseases and the rising number of organ and stem cell transplant procedures worldwide are major drivers for the Photopheresis Products market
- For instance, increasing cases of graft-versus-host disease following hematopoietic stem cell transplantation are driving demand for photopheresis as an effective second-line or adjunctive therapy
- Photopheresis is gaining clinical acceptance due to its immunomodulatory mechanism, which helps manage disease progression while minimizing long-term immunosuppression risks
- Furthermore, expanding transplant programs, particularly in North America, Europe, and parts of Asia Pacific, are increasing the utilization of photopheresis systems and disposable kits
- Growing awareness among clinicians regarding the long-term benefits of photopheresis in managing chronic immune conditions is further supporting adoption across hospitals and specialty treatment centers
- Collectively, these factors are driving sustained demand for photopheresis products across both established and emerging healthcare markets
Restraint/Challenge
High Treatment Costs and Limited Availability of Specialized Infrastructure
- High costs associated with photopheresis systems, consumables, and treatment procedures pose a significant challenge to market expansion
- For instance, the need for specialized equipment, trained healthcare professionals, and dedicated treatment centres limits widespread adoption in low- and middle-income regions
- In addition, photopheresis procedures are time-intensive and often require multiple treatment sessions, which can increase the overall cost burden for healthcare providers and patients
- Limited reimbursement coverage in certain countries and regions further restricts patient access to photopheresis therapy
- The lack of awareness and limited clinical expertise in some healthcare systems also slows adoption, particularly outside major tertiary care hospitals
- Overcoming these challenges through improved reimbursement policies, cost-effective product development, and expanded clinician training will be critical for sustained growth of the global photopheresis products market
Photopheresis Products Market Scope
The market is segmented on the basis of product type, application, and end user.
- By Product Type
On the basis of product type, the Photopheresis Products market is segmented into open system and closed system. The closed system segment dominated the largest market revenue share of 64.8% in 2025, driven by its superior safety profile, reduced risk of contamination, and higher level of automation. Closed systems are widely preferred in hospital and specialty clinic settings as they ensure controlled blood handling and comply with stringent regulatory and infection-control standards. These systems integrate leukapheresis, photoactivation, and reinfusion into a single workflow, improving procedural efficiency. Increasing adoption in transplant centers and oncology clinics further supports dominance. Closed systems also reduce operator dependency and procedural variability, enhancing treatment consistency. Strong regulatory approvals and growing clinical acceptance in GVHD and CTCL treatment contribute to revenue leadership. In addition, higher reimbursement acceptance for closed systems supports sustained demand.
The open system segment is expected to witness the fastest CAGR of 9.6% from 2026 to 2033, driven by lower equipment costs and flexibility in procedural customization. Open systems are increasingly adopted in emerging markets where budget constraints influence purchasing decisions. These systems allow healthcare providers to utilize existing apheresis equipment, reducing capital investment. Growing healthcare infrastructure development and rising awareness of photopheresis therapy in developing regions support growth. Technological improvements addressing contamination risks are also improving adoption. Expansion of treatment indications further fuels demand. Overall, open systems are gaining traction due to affordability and adaptability.
- By Application
On the basis of application, the Photopheresis Products market is segmented into graft versus host disease (GVHD), cutaneous T-cell lymphoma (CTCL), transplant rejection, autoimmune diseases, and others. The GVHD segment dominated the market with a revenue share of 38.5% in 2025, supported by the increasing number of hematopoietic stem cell transplants globally. Photopheresis is widely recognized as an effective second-line therapy for steroid-refractory GVHD. Rising transplant procedures, improved survival rates, and growing clinical evidence supporting photopheresis efficacy drive dominance. Hospitals increasingly integrate photopheresis into standard GVHD treatment protocols. Favorable safety profile and long-term disease control further enhance adoption. Regulatory support and clinical guideline inclusion strengthen market leadership. Increasing investments in transplant care also support sustained demand.
The autoimmune diseases segment is projected to register the fastest CAGR of 10.2% from 2026 to 2033, driven by expanding clinical research and growing off-label use of photopheresis. Increasing prevalence of autoimmune disorders and limitations of conventional immunosuppressive therapies are accelerating adoption. Photopheresis offers targeted immune modulation with fewer systemic side effects, making it attractive for long-term disease management. Ongoing clinical trials exploring new indications further boost growth. Rising physician awareness and patient acceptance support expansion. Improved reimbursement frameworks are also contributing to faster uptake.
- By End User
On the basis of end user, the Photopheresis Products market is segmented into hospitals, specialty clinics, ambulatory care centers, and others. The hospitals segment accounted for the largest market revenue share of 56.9% in 2025, driven by the availability of advanced infrastructure and trained medical professionals. Hospitals handle a high volume of transplant and oncology patients requiring photopheresis therapy. Integration of photopheresis units within hospital blood banks and oncology departments supports dominance. Strong reimbursement coverage and access to multidisciplinary care further enhance adoption. Hospitals also lead in clinical research and adoption of new photopheresis technologies. Increasing patient inflow and complex case management reinforce revenue leadership.
The specialty clinics segment is expected to witness the fastest CAGR of 8.8% from 2026 to 2033, fueled by the shift toward outpatient and specialized care models. Specialty clinics offer focused expertise, reduced treatment costs, and improved patient convenience. Growing investments in specialty immunology and oncology centers support market growth. Increasing decentralization of photopheresis services from hospitals to clinics further accelerates adoption. Technological advancements enabling compact and clinic-friendly systems also contribute. Overall, specialty clinics are emerging as high-growth end users due to efficiency and patient-centric care delivery.
Photopheresis Products Market Regional Analysis
- North America dominated the photopheresis products market with the largest revenue share of 41.8% in 2025, supported by advanced healthcare infrastructure, high adoption of specialty and immunomodulatory therapies, and the strong presence of leading photopheresis system manufacturers
- Healthcare providers in the region highly value the clinical efficacy, safety profile, and immune-modulating benefits of photopheresis in treating conditions such as graft versus host disease (GVHD) and cutaneous T-cell lymphoma (CTCL)
- This widespread adoption is further supported by favorable reimbursement policies, high awareness among clinicians, well-established transplant and oncology centers, and increasing utilization of photopheresis as a second-line therapy, establishing it as a standard treatment option across major hospitals and specialty clinics
U.S. Photopheresis Products Market Insight
The U.S. photopheresis products market captured the largest revenue share of 81% in 2025 within North America, driven by the high volume of hematopoietic stem cell transplants, strong adoption of advanced immunotherapies, and early acceptance of photopheresis in clinical practice. The presence of leading manufacturers, extensive clinical research activity, and widespread availability of specialized treatment centers significantly support market growth. Increasing use of photopheresis in oncology, transplant rejection management, and autoimmune disorders further accelerates demand. In addition, supportive reimbursement frameworks and continuous technological advancements contribute to sustained market expansion in the U.S.
Europe Photopheresis Products Market Insight
The Europe photopheresis products market is projected to expand at a substantial CAGR throughout the forecast period, driven by rising adoption of advanced immunomodulatory therapies and increasing prevalence of hematological malignancies and autoimmune diseases. Strong regulatory frameworks, expanding transplant programs, and growing awareness of photopheresis benefits among clinicians support market growth. The region is witnessing increased utilization of photopheresis across hospitals and specialty clinics, particularly for GVHD and CTCL management. Ongoing clinical studies and improvements in healthcare access further contribute to market expansion.
U.K. Photopheresis Products Market Insight
The U.K. photopheresis products market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by the National Health Service’s (NHS) focus on advanced specialty treatments and improved access to immunotherapy services. Increasing stem cell transplant procedures and rising awareness of photopheresis as an effective second-line therapy are key growth drivers. The expansion of specialized oncology and transplant centers further supports adoption. In addition, growing clinical evidence and standardized treatment protocols are strengthening market penetration in the U.K.
Germany Photopheresis Products Market Insight
The Germany photopheresis products market is expected to expand at a considerable CAGR during the forecast period, driven by strong healthcare infrastructure, high investment in advanced medical technologies, and a growing emphasis on evidence-based therapies. Germany’s leadership in clinical research and transplant medicine supports increasing adoption of photopheresis systems. The rising prevalence of autoimmune and hematological disorders, combined with favorable reimbursement policies, is accelerating market growth. Hospitals and specialty clinics are increasingly integrating photopheresis into treatment pathways.
Asia-Pacific Photopheresis Products Market Insight
The Asia-Pacific photopheresis products market is expected to grow at the fastest CAGR during the forecast period, driven by expanding healthcare infrastructure, increasing awareness of advanced immunotherapies, and rising prevalence of autoimmune and hematological diseases. Growing investments in specialty treatment facilities and transplant programs across emerging economies are significantly boosting adoption. Improving access to advanced medical technologies and increasing physician awareness further support rapid market expansion across the region.
Japan Photopheresis Products Market Insight
The Japan photopheresis products market is gaining momentum due to advanced healthcare systems, strong clinical research capabilities, and increasing adoption of innovative immunomodulatory treatments. Rising incidence of hematological disorders and transplant procedures is driving demand for photopheresis therapy. The country’s emphasis on precision medicine and long-term disease management supports market growth. In addition, an aging population and increasing focus on minimally invasive therapies further contribute to adoption.
China Photopheresis Products Market Insight
The China photopheresis products market accounted for the largest revenue share in Asia Pacific in 2025, driven by rapid expansion of healthcare infrastructure, increasing awareness of advanced immunotherapies, and rising prevalence of autoimmune and hematological disorders. Growing investments in specialty hospitals and transplant centers are accelerating adoption of photopheresis systems. Supportive government initiatives to modernize healthcare services and improve access to advanced treatments further propel market growth in China.
Photopheresis Products Market Share
The Photopheresis Products industry is primarily led by well-established companies, including:
• Fresenius Kabi (Germany)
• Macopharma (France)
• PIT Medical Systems (Germany)
• Haemonetics Corporation (U.S.)
• Cerus Corporation (U.S.)
• Asahi Kasei Medical (Japan)
• Terumo BCT (Japan)
• Kawasumi Laboratories (Japan)
• Nikkiso Co., Ltd. (Japan)
• Medtronic (Ireland)
• B. Braun Melsungen (Germany)
• Baxter International (U.S.)
• JMS Co., Ltd. (Japan)
• HemaCare Corporation (U.S.)
• Miltenyi Biotec (Germany)
• Sartorius AG (Germany)
• Bio-Rad Laboratories (U.S.)
• Grifols (Spain)
Latest Developments in Global Photopheresis Products Market
- In February 2024, Mallinckrodt plc presented real-world outcomes data on its THERAKOS™ CELLEX Photopheresis System at the 2024 Tandem Meetings, showing improvements in overall survival and response rates for patients treated for steroid-refractory chronic graft-versus-host disease, underscoring clinical validation and adoption of photopheresis technology
- In September 2024, Mallinckrodt plc announced that the THERAKOS CELLEX Photopheresis System received CE certification under the updated European Union Medical Device Regulation (EU MDR) 2017/745, authorizing its continued use across the EU for patient indications including cutaneous T-cell lymphoma and graft-versus-host disease, demonstrating regulatory progress for established photopheresis platforms
- In April 2023, Fresenius Kabi launched a single-needle venous access option for its Amicus® Extracorporeal Photopheresis (ECP) System at the European Society for Blood and Marrow Transplantation meeting, which obtained CE marking for treatment of cutaneous T-cell lymphoma unresponsive to other therapies, representing a product enhancement that can improve procedural flexibility and patient experience
- In August 2024, Mallinckrodt plc and CVC Capital Partners entered into a definitive agreement under which CVC Capital Partners Fund IX would acquire the Therakos business for approximately $925 million, subject to customary adjustments, indicating significant investment and consolidation activity in the photopheresis technology space
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.