Global Plant Based Vaccine Production Market
Market Size in USD Million
CAGR :
% 
 USD
833.09 Million
USD
2,548.44 Million
2024
2032
USD
833.09 Million
USD
2,548.44 Million
2024
2032
| 2025 –2032 | |
| USD 833.09 Million | |
| USD 2,548.44 Million | |
|
|
|
|
Plant-Based Vaccine Production Market Size
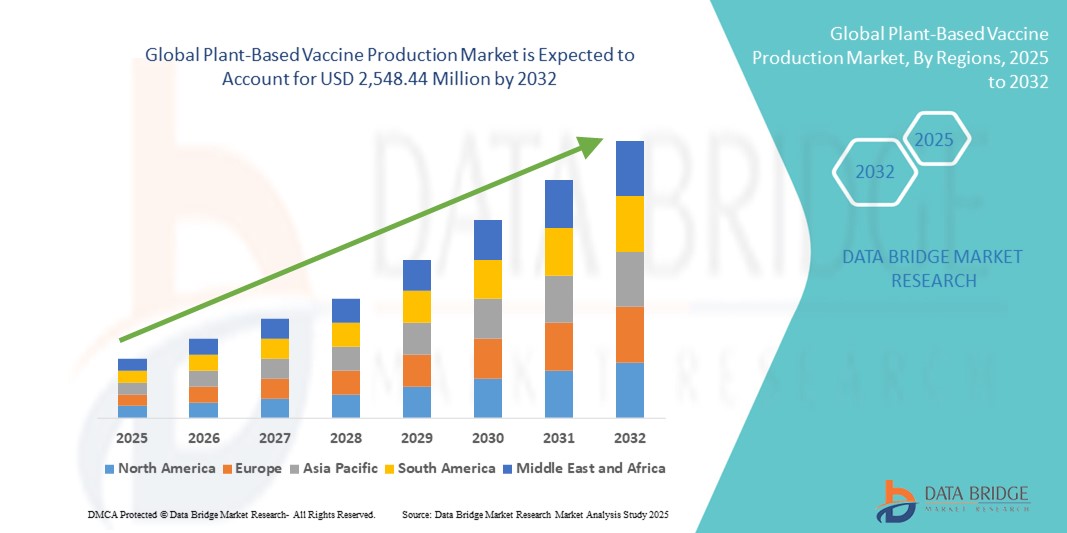
- The global plant-based vaccine production market size was valued at USD 833.09 million in 2024 and is expected to reach USD 2,548.44 million by 2032, at a CAGR of 15.00% during the forecast period
- The market growth is largely fueled by increasing investment in biotechnology research, advancements in plant genetic engineering, and the growing need for scalable, safe, and cost-effective vaccine manufacturing alternatives. Plant-based systems offer significant advantages over traditional platforms, including reduced risk of contamination with human pathogens and faster response times during outbreaks
- Furthermore, rising global demand for vaccines—particularly in low- and middle-income countries—has accelerated interest in plant-derived vaccines due to their potential for rapid, large-scale production. These converging factors are accelerating the uptake of Plant-Based Vaccine Production solutions, thereby significantly boosting the industry's growth
Plant-Based Vaccine Production Market Analysis
- Plant-based vaccine production, leveraging genetically engineered plants to express immunogenic proteins, is emerging as a transformative biotechnological approach, offering scalable, cost-effective, and safer alternatives to traditional vaccine manufacturing methods
- The increasing global focus on pandemic preparedness, demand for rapid and low-cost vaccine production platforms, and the ability of plant-based systems to reduce contamination risks are primary factors propelling market growth
- North America dominated the plant-based vaccine production market with the largest revenue share of 44.98% in 2024, supported by strong R&D infrastructure, favorable regulatory policies, and the presence of key players such as Kentucky BioProcessing and Medicago. The U.S. is witnessing significant developments in this space with accelerated government funding and biotech collaborations
- Asia-Pacific is projected to be the fastest-growing region in the plant-based vaccine production market over the forecast period, driven by increased biotech investments, rising awareness about alternative vaccines, and government support for indigenous vaccine manufacturing in countries like China and India
- The transient expression segment dominated the plant-based vaccine production market with a market share of 42.5% in 2024, due to its rapid protein production capabilities and scalability for emergency vaccine development
Report Scope and Plant-Based Vaccine Production Market Segmentation
|
Attributes |
Plant-Based Vaccine Production Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Plant-Based Vaccine Production Market Trends
Growing Need Due to Demand for Cost-Effective and Scalable Vaccine Platforms
- The increasing demand for affordable, scalable, and rapid vaccine development platforms—especially in response to pandemics and emerging infectious diseases—is significantly driving growth in the global plant-based vaccine production market
- For instance, in April 2024, Medicago Inc. (a pioneer in plant-based vaccines) announced its advancement in virus-like particle (VLP) production using Nicotiana benthamiana, showcasing faster response times compared to traditional egg-based systems. Such innovations are positioning plant-based platforms as critical in future outbreak preparedness
- Plant-based vaccine production allows for rapid protein expression, lower production costs, and minimal risk of contamination by animal pathogens—making it highly attractive for both human and veterinary vaccine development
- Furthermore, the increasing prevalence of zoonotic and infectious diseases, including influenza, COVID-19 variants, and dengue, is prompting governments and biotech firms to invest in alternative production systems like molecular farming in plants
- Plant-based vaccines are also gaining attention for their potential in developing oral or edible vaccines, which can improve compliance, reduce cold chain logistics, and simplify mass immunization campaigns in resource-limited settings
Plant-Based Vaccine Production Market Dynamics
Driver
Biopharming and Molecular Farming Redefining Vaccine Manufacturing
- A significant and accelerating trend in the global Plant-Based Vaccine Production market is the rapid advancement of biopharming technologies, particularly the use of genetically engineered plants to produce pharmaceutical proteins. This technique, often referred to as molecular farming, is revolutionizing vaccine manufacturing by enabling scalable, cost-effective, and rapid-response production platforms
- For instance, companies like Medicago Inc. have pioneered the use of Nicotiana benthamiana (a relative of tobacco) to develop virus-like particle (VLP) vaccines, including COVID-19 vaccines, showcasing the speed and flexibility of plant-based systems in responding to global health crises
- Unlike traditional vaccine production methods that rely on eggs or mammalian cells, plant-based platforms offer several advantages, such as reduced contamination risk, lower production costs, and shorter development timelines. These benefits are particularly critical during pandemics or emerging infectious disease outbreaks
- The increasing investment from both public and private sectors in plant-derived biopharmaceuticals has catalyzed R&D, leading to novel applications beyond infectious diseases, including cancer immunotherapy and metabolic disorder treatments
- Furthermore, regulatory agencies such as the FDA and EMA are beginning to establish clearer guidelines for plant-based biologics, paving the way for faster approvals and wider adoption. This is encouraging more biotech firms and pharmaceutical companies to explore plant-based vaccine pipelines
- The demand for safe, scalable, and globally accessible vaccine manufacturing solutions is driving widespread interest in this technology, especially in low- and middle-income countries where cold chain limitations and production bottlenecks have historically hindered immunization campaigns
Restraint/Challenge
Regulatory Uncertainty and Limited Commercial-Scale Infrastructure
- Despite their promise, plant-based vaccines face significant regulatory and infrastructure-related hurdles that slow commercialization and market acceptance. Unlike traditional vaccine platforms, plant-based systems still lack standardized global regulatory frameworks for fast-track approvals
- For instance, while Canada approved Medicago's Covifenz COVID-19 vaccine in 2022, the absence of FDA or EMA approval reflects challenges in regulatory alignment and data harmonization across regions
- Additionally, the high upfront investment needed to establish GMP-compliant greenhouses or vertical farms for plant-based production limits entry for smaller firms and slows scalability
- Another restraint is the relatively limited clinical data available for plant-based vaccines compared to established platforms, making stakeholders cautious in mass deployment
- Addressing these issues will require coordinated efforts between regulatory bodies, industry stakeholders, and research institutions to develop harmonized guidelines, incentivize infrastructure investment, and conduct broader clinical validations
Plant-Based Vaccine Production Market Scope
The market is segmented on the basis of type, expression system, technology, and application.
- By Type
On the basis of type, the plant-based vaccine production market is segmented into viral vaccines, bacterial vaccines, parasitic vaccines, cancer vaccines, and others. The viral vaccines segment dominated with the largest market revenue share of 36.8% in 2024, driven by the increasing incidence of viral outbreaks and the urgent need for rapid, scalable vaccine development platforms.
The cancer vaccines segment is projected to grow at the fastest CAGR of 12.9% from 2025 to 2032, fueled by rising demand for targeted immunotherapy and innovations in plant-derived antigen delivery systems.
- By Expression System
On the basis of expression system, the plant-based vaccine production market is segmented into nuclear transformation, chloroplast transformation, transient expression, stable expression, and others. The transient expression segment held the largest market share of 42.5% in 2024, due to its rapid protein production capabilities and scalability for emergency vaccine development.
The chloroplast transformation segment is expected to witness the highest CAGR of 11.8% during the forecast period, attributed to its cost-effectiveness, high expression levels, and biosafety advantages.
- By Technology
On the basis of technology, the plant-based vaccine production market is segmented into recombinant subunit vaccines, virus-like particle (VLP) vaccines, edible vaccines, live attenuated vaccines, and others. The recombinant subunit vaccines segment accounted for the largest market share of 34.2% in 2024, driven by increasing adoption in both human and veterinary immunization programs due to safety and precision.
The virus-like particle (VLP) vaccines segment is forecast to grow at the fastest CAGR of 13.1% from 2025 to 2032, supported by their proven immunogenicity and ability to mimic native viral structures without the risk of infection.
- By Application
On the basis of application, the plant-based vaccine production market is segmented into human use, veterinary use, research & development, diagnostic use, and others. The human use segment held the dominant share of 49.6% in 2024, supported by the growing need for scalable and affordable vaccine platforms in developing and developed regions.
The veterinary use segment is projected to grow at the fastest CAGR of 10.7% over the forecast period, driven by increasing investments in livestock health, zoonotic disease prevention, and plant-based immunization strategies for animals.
Plant-Based Vaccine Production Market Regional Analysis
- North America dominated the plant-based vaccine production market with the largest revenue share of 44.98% in 2024, driven by increasing investments in biotechnology, advancements in molecular farming techniques, and strong support from regulatory agencies for alternative vaccine platforms
- The region’s robust pharmaceutical infrastructure and active government funding for pandemic preparedness initiatives have accelerated R&D in plant-based vaccine development. In addition, collaborations between public health organizations and biotech firms are further driving innovation and commercialization
- North America also benefits from a high concentration of leading companies like Medicago Inc. and Kentucky Bioprocessing, which are pioneering breakthroughs in virus-like particles (VLPs) using plant expression systems
U.S. Plant-Based Vaccine Production Market Insight
The U.S. plant-based vaccine production market captured the largest revenue share of 65% in 2024 within North America, driven by extensive investments in biopharmaceutical R&D, growing government initiatives for vaccine innovation, and the country’s advanced regulatory framework that supports clinical trials and emergency use approvals. The adoption of plant-based systems is increasing due to their speed, cost-efficiency, and safety compared to traditional platforms. Notable developments, such as DARPA’s funding for plant-based countermeasures and Outbreak Response programs, highlight the strategic importance of this market in U.S. healthcare policy.
Europe Plant-Based Vaccine Production Market Insight
The Europe plant-based vaccine production market is projected to grow at a substantial CAGR over the forecast period, fueled by increasing demand for sustainable vaccine technologies and strong support from EU funding programs for biotech innovation. Regulatory advances in countries like Germany and France, along with increased public-private partnerships, are fostering a more favorable environment for commercialization. Europe’s focus on pandemic readiness and alternative platforms for influenza and COVID-19 vaccines are key drivers.
U.K. Plant-Based Vaccine Production Market Insight
The U.K. plant-based vaccine production market is anticipated to grow at a noteworthy CAGR, supported by investments in synthetic biology and pharmaceutical R&D. The U.K. government’s support for molecular farming projects and plant-based biotechnology through agencies like Innovate UK is accelerating pilot-scale and clinical developments. Moreover, academic collaborations with biotech startups are aiding in the discovery of novel antigens and cost-effective production systems, with future applications targeting both human and veterinary vaccines.
Germany Plant-Based Vaccine Production Market Insight
The Germany plant-based vaccine production market is expanding considerably, supported by the country’s reputation for biopharma manufacturing excellence, sustainability initiatives, and innovation-driven research programs. Germany is emerging as a key hub for molecular bioprocessing and plant-derived therapeutics, with growing public interest in eco-conscious vaccine alternatives. Academic institutions and government-funded research are playing a vital role in supporting early-stage development and scale-up.
Asia-Pacific Plant-Based Vaccine Production Market Insight
The Asia-Pacific plant-based vaccine production market is expected to grow at the fastest CAGR from 2025 to 2032, driven by expanding biotech investments, rising demand for affordable healthcare, and government-led initiatives in countries like India, China, and Japan. The region is witnessing an increase in partnerships between research institutes and pharmaceutical firms aimed at exploring novel plant-based vaccine candidates, particularly for regional diseases like dengue, rabies, and Japanese encephalitis.
Japan Plant-Based Vaccine Production Market Insight
The Japan plant-based vaccine production market is gaining momentum with its focus on high-tech solutions and innovative biomanufacturing. The country is investing in novel expression systems using plants such as rice and tobacco, driven by its aging population and demand for next-generation vaccines. Japanese biotech companies are working alongside public institutions to develop safe, effective, and scalable solutions that cater to both domestic and global health challenges.
China Plant-Based Vaccine Production Market Insight
The China plant-based vaccine production market accounted for the largest market revenue share in Asia-Pacific in 2024, driven by large-scale government investment in biotech innovation and efforts to reduce reliance on imported vaccines. The country's push toward smart agriculture and synthetic biology is benefiting plant-based vaccine production, and local firms are investing heavily in creating GMP-compliant greenhouses and expression systems to meet domestic and export demand.
Plant-Based Vaccine Production Market Share
The plant-based vaccine production industry is primarily led by well-established companies, including:
- Medicago Inc. (Canada)
- Kentucky BioProcessing, Inc. (U.S.)
- iBio, Inc. (U.S.)
- Leaf Expression Systems Ltd (U.K.)
- ICON Genetics GmbH (Germany)
- Protalix Biotherapeutics (Israel)
- Cape Bio Pharms (South Africa)
- PhytoAB Inc. (U.S.)
- BAT (British American Tobacco) via KBP (U.S.)
- Zyus Life Sciences (Canada)
Latest Developments in Global Plant-Based Vaccine Production Market
- In May 2025, Sanofi announced the acquisition of Vicebio, a London-based biotech firm backed by the University of Queensland’s Molecular Clamp technology platform, for approximately USD 2 billion. This strategic move aims to accelerate the development of next-generation plant-based vaccines targeting multiple pathogens such as RSV and influenza. The acquisition underscores Sanofi’s commitment to innovative vaccine platforms and is expected to significantly advance the global plant-based vaccine production landscape
- In May 2025, BioNTech SE secured up to USD 145 million in funding from the Coalition for Epidemic Preparedness Innovations (CEPI) to establish Africa’s first mRNA-based vaccine manufacturing facility in Kigali, Rwanda. While the primary focus is on mRNA, the initiative supports the broader mission of scalable, locally manufactured vaccines including plant-based platforms. This development is set to boost regional production capabilities and enhance preparedness against endemic and emerging diseases
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.