Global Plasma Protease C1 Inhibitor Market
Market Size in USD Million
CAGR :
% 
 USD
909.85 Million
USD
1,634.81 Million
2024
2032
USD
909.85 Million
USD
1,634.81 Million
2024
2032
| 2025 –2032 | |
| USD 909.85 Million | |
| USD 1,634.81 Million | |
|
|
|
|
Plasma Protease C1-inhibitor Market Analysis
The Plasma Protease C1-Inhibitor market is centered around therapies aimed at addressing deficiencies or dysfunctions in the C1-inhibitor (C1-INH) protein in patients with hereditary angioedema (HAE). HAE is a rare, inherited disorder characterized by unpredictable episodes of swelling, often affecting the extremities, gastrointestinal tract, and airways, which can be life-threatening if not managed appropriately. The increasing global incidence of HAE is a key driver which is flourishing the market growth, as the condition remains underdiagnosed and often misdiagnosed. As awareness and diagnostic capabilities improve, more individuals are being diagnosed, leading to a rising demand for effective treatment options.
The primary goal of C1-INH therapies is to restore the deficient or dysfunctional protein to prevent and manage acute attacks and to prevent the recurrence of swelling episodes. Recent advancements in both plasma-derived and recombinant C1-INH therapies have enhanced treatment efficacy, further fueling demand. Additionally, the growing emphasis on personalized medicine and more targeted treatments has encouraged the development of new therapies with fewer side effects and greater long-term benefits. As awareness, diagnosis, and treatment options improve, the Plasma Protease C1-Inhibitor market is expected to experience continued expansion throughout the forecast period.
Plasma Protease C1-inhibitor Market Size
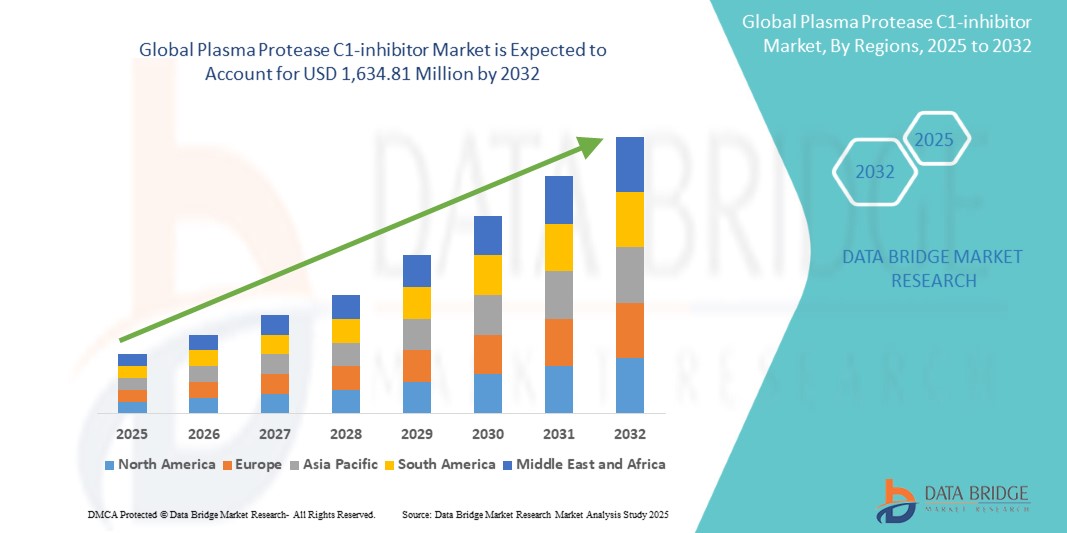
The global plasma protease C1-inhibitor market size was valued at USD 909.85 million in 2024 and is projected to reach 1,634.81 million by 2032, with a CAGR of 7.60% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Plasma Protease C1-inhibitor Market Trends
“Shift from Plasma-Derived to Recombinant C1-INH Products”
Recombinant C1-INH therapies are gaining traction due to their potential for higher purity and consistency compared to plasma-derived alternatives. Recombinant therapies also carry a lower risk of bloodborne pathogen transmission, which is a significant concern for plasma-based products. This shift is influencing the market as patients and healthcare providers opt for the safety and reliability of recombinant therapies. Leading companies are focusing on the development and commercialization of recombinant C1-INH products, which are seen as the future of treatment for hereditary angioedema and other complement-related disorders.
Report Scope and Plasma Protease C1-inhibitor Market Segmentation
|
Attributes |
Plasma Protease C1-inhibitor Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Italy, Spain, Netherlands, Russia, Denmark, Norway, Rest of Europe in Europe, China, Japan, India, Malaysia, South Korea, Australia, Malaysia, Thailand, Vietnam, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Kuwait, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Key Market Players |
BioCryst Pharmaceuticals, Inc. (U.S.), CENTOGENE N.V. (Germany), CSL (Australia), Ionis Pharmaceuticals (U.S.), KalVista Pharmaceuticals (U.S.), Pharming (The Netherlands), and Takeda Pharmaceutical Company Limited (Japan) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Plasma Protease C1-inhibitor Market Definition
The Plasma Protease C1-Inhibitor (C1-INH) is a critical protein involved in regulating the immune system, specifically the complement system, which plays a vital role in the body's defense against infections. In conditions such as hereditary angioedema (HAE), a rare genetic disorder, there is a deficiency or dysfunction of C1-INH, leading to uncontrolled activation of the complement system and recurrent episodes of swelling (angioedema), which can be potentially life-threatening. Plasma-derived and recombinant C1-INH therapies are the primary treatments for managing acute and prophylactic HAE attacks.
Plasma Protease C1-inhibitor Market Dynamics
Drivers
- Rising Prevalence of Hereditary Angioedema (HAE)
The increasing global incidence of hereditary angioedema (HAE), a rare genetic disorder caused by a deficiency or dysfunction of the C1-inhibitor protein, is a primary driver of the market. As awareness and diagnosis of HAE improve, there is a growing demand for effective therapies to manage acute attacks and prevent recurrence, boosting the demand for C1-inhibitor products. Globally, it is estimated one in 50,000 people have hereditary angioedema, however, because it is rare, it is often not correctly diagnosed. The rising prevalence of HAE, coupled with advancements in treatment options and increasing awareness, is expected to significantly fuel the growth of the Plasma Protease C1-Inhibitor market in the coming years, offering new opportunities for both patients and market players.
- Advancements in C1-Inhibitor Therapy
The development of novel recombinant human C1-inhibitor therapies and improvements in plasma-derived therapies are contributing to the growth of the market. These advanced treatments offer higher safety, efficacy, and convenience compared to traditional therapies, leading to better patient outcomes and higher adoption rates among healthcare providers. A study published in Allergy, Asthma & Clinical Immunology found that long-term prophylaxis treatment with subcutaneous C1-inhibitors effectively helps patients achieve disease control and secure a better quality of life in hereditary angioedema (HAE). The continuous innovation in C1-inhibitor therapies is expected to drive the market's expansion, making treatment more accessible, effective, and patient-friendly, while encouraging broader adoption across healthcare systems globally.
Opportunities
- Emergence of Gene Therapy for HAE
One of the most promising opportunities in the C1-Inhibitor market is the development of gene therapy for hereditary angioedema (HAE). Gene therapy offers the potential for a long-term or permanent solution by addressing the root cause of C1-INH deficiency. Successful gene therapies could revolutionize the treatment landscape, potentially reducing or eliminating the need for lifelong therapies and opening new avenues for market growth. In conclusion, the advancement of gene therapy represents a transformative opportunity for the C1-Inhibitor market, offering the potential to significantly improve patient outcomes, reduce treatment burdens, and create a new era of curative treatments, thereby driving market expansion and innovation.
- Opportunities in Non-HAE Applications
Plasma protease C1-inhibitors hold considerable potential for treating a range of disorders linked to dysfunctions in the complement system beyond hereditary angioedema (HAE), The complement system plays a significant role in inflammation, immune responses, and the defense against pathogens in conditions including systemic lupus erythematosus, rheumatoid arthritis rare genetic disorders and others. Abnormalities in the complement cascade can lead to several autoimmune, inflammatory, and other related diseases. Expanding the therapeutic scope of C1-inhibitors to these conditions could unlock substantial market opportunities.
Restraints/Challenges
- High Treatment Costs
One of the primary challenges in the C1-Inhibitor market is the high cost of therapies. Plasma-derived and recombinant C1-INH treatments are expensive, making them less accessible to patients, especially in low and middle-income countries. The financial burden of long-term treatment, which may continue throughout a patient's life, can limit patient access to these essential therapies, thus restricting market growth in some regions. In conclusion, the high cost of C1-Inhibitor treatments remains a significant restraint on market growth, particularly in emerging economies.
- Limited Patient Population (Rare Disease)
The market for C1-Inhibitor therapies is primarily driven by the treatment of hereditary angioedema (HAE), a rare disease affecting a small patient population. The limited number of patients can make it challenging to justify large-scale production or significant investments in R&D for new therapies, potentially slowing innovation and market expansion. In conclusion, the small patient population for HAE presents a challenge for market growth, as it limits economies of scale and may deter investment in research and development.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Plasma Protease C1-inhibitor Market Scope
The market is segmented on the basis of drug class, dosage form and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Drug Class
- C1-inhibitors
- C1-esterase Inhibitor
- Recombinant Inhibitor
- Kallikrein Inhibitor
- Selective Bradykinin B2 Receptor Antagonist
Dosage Form
- Lyophilised
- Injectables
Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Plasma Protease C1-inhibitor Market Regional Analysis
The market is analysed and market size insights and trends are provided by drug class, dosage form and distribution channel as referenced above.
The countries covered in the market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Italy, Spain, Netherlands, Russia, Denmark, Norway, Rest of Europe in Europe, China, Japan, India, Malaysia, South Korea, Australia, Malaysia, Thailand, Vietnam, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Kuwait, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
The North American plasma protease C1-inhibitor market maintains a dominant position, largely due to the high prevalence of hereditary angioedema (HAE), which creates strong demand for effective treatment options. This demand contributes substantially to the market's expansion. Additionally, North America benefits from a well-established healthcare infrastructure and a favorable regulatory environment, which accelerates the approval process and supports the widespread adoption of new therapies. For instance, in June 2022, ORLADEYO (berotralstat) was approved as the first oral, once-daily prophylactic treatment for preventing swelling attacks in patients with HAE aged 12 years and older.
The plasma protease C1-inhibitors market in the Asia-Pacific region is seeing notable growth during the forecast period, fueled by an increasing prevalence of chronic inflammatory diseases and a rising demand for advanced treatment solutions. Key factors fueling this expansion include the introduction of novel recombinant human inhibitors, which offer improved safety and effectiveness compared to traditional therapies. Additionally, the region's aging population is leading to a higher incidence of age-related conditions that can benefit from C1-inhibitor therapies, further contributing to market growth.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Plasma Protease C1-inhibitor Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Plasma Protease C1-inhibitor Market Leaders Operating in the Market Are:
- BioCryst Pharmaceuticals, Inc. (U.S.)
- CENTOGENE N.V. (Germany)
- CSL (Australia)
- Ionis Pharmaceuticals (U.S.)
- KalVista Pharmaceuticals (U.S.)
- Pharming (The Netherlands)
- Takeda Pharmaceutical Company Limited (Japan)
Latest Developments in Plasma Protease C1-inhibitor Market
- In June 2024, BioCryst Pharmaceuticals, Inc. presented new evidence indicating that patients with hereditary angioedema (HAE) who have normal C1-inhibitor levels and function (HAE-nC1-INH) experienced a reduction in monthly attack rates after initiating treatment with oral, once-daily ORLADEYO (berotralstat). The analysis included six patients with HAE-nC1-INH. After six months of treatment with berotralstat, five patients showed a 75 to 100 percent reduction in their HAE attack rate, while one patient, who was also taking tranexamic acid, experienced a 29 percent reduction in attack frequency
- In May 2024, KalVista Pharmaceuticals, Inc. outlined its strategic plans for fiscal year 2025, starting on May 1. These plans include the development of sebetralstat, the company’s investigational oral plasma kallikrein inhibitor for on-demand treatment of hereditary angioedema (HAE). The strategy involves submitting multiple regulatory filings for sebetralstat and preparing for its swift commercialization upon approval
- In January 2024, Ionis announced positive topline results from the Phase 3 OASIS-HAE study of the investigational drug donidalorsen in patients with hereditary angioedema. The trial successfully met its primary endpoint, demonstrating a significant reduction in the rate of angioedema attacks in patients treated with donidalorsen (80 mg) via subcutaneous injection every 4 weeks (Q4W) (p<0.001) or every 8 weeks (Q8W) (p=0.004), compared to placebo. Additionally, donidalorsen achieved statistical significance on all secondary endpoints in the Q4W group and key secondary endpoints in the Q8W group
- In February 2023, Takeda announced that the U.S. Food and Drug Administration (FDA) has approved the supplemental Biologics License Application (sBLA) for the expanded use of TAKHZYRO (lanadelumab-flyo) for prophylaxis to prevent hereditary angioedema (HAE) attacks in pediatric patients aged 2 to <12 years. Prior to this approval, the only available routine prophylaxis treatments for children aged 6 to <12 required dosing every three to four days, while no approved prophylaxis options existed for children aged 2 to <6. With this approval, TAKHZYRO becomes the first prophylactic treatment for this younger age group
- In December 2022, Takeda introduced Cinryze in India for the treatment of hereditary angioedema. Cinryze is approved for routine prevention (prophylaxis) of angioedema, as well as for the treatment of acute angioedema attacks and pre-procedure prevention. The launch aims to address acute HAE attacks and reduce the frequency of future episodes through prophylactic therapy, ultimately improving the quality of life for individuals with HAE
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.