Global Plexiform Neurofibromas Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
1.45 Billion
USD
2.74 Billion
2024
2032
USD
1.45 Billion
USD
2.74 Billion
2024
2032
| 2025 –2032 | |
| USD 1.45 Billion | |
| USD 2.74 Billion | |
|
|
|
|
Plexiform Neurofibromas Treatment Market Size
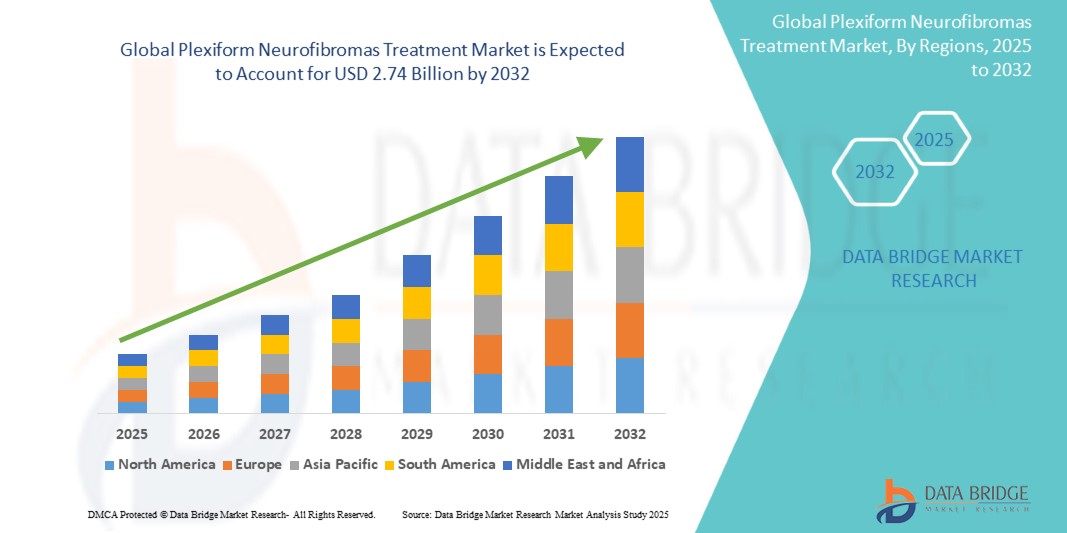
- The Global Plexiform Neurofibromas Treatment Market size was valued at USD 1.45 billion in 2024 and is expected to reach USD 2.74 billion by 2032, at a CAGR of 8.3% during the forecast period
- This growth is driven by factors such as increasing prevalence of plexiform neurofibromas, advancements in diagnostic techniques, rising awareness among patients and healthcare providers, and the growing demand for targeted and minimally invasive treatment options.
Plexiform Neurofibromas Treatment Market Analysis
- Plexiform neurofibromas are inherited genetic disorders resulting in developments of neurofibromas from multiple nerves and skin. Soft lumps on and under the skin are the common symptoms of plexiform neurofibromas. Plexiform neurofibromas result in increasing the chances of cancer in the patients
- The demand for Plexiform Neurofibromas Treatment is significantly driven by the increasing prevalence of neurofibromatosis type 1 (NF1), heightened awareness through patient advocacy and early diagnosis programs, and technological advancements in targeted therapies and minimally invasive treatment approaches.
- North America is expected to dominate the Plexiform Neurofibromas Treatment market with a share of 36.4%, attributed to its advanced healthcare infrastructure, higher adoption of innovative therapeutic modalities, and the strong presence of leading biopharmaceutical companies and research institutions.
- Asia-Pacific is projected to be the fastest-growing region in the Plexiform Neurofibromas Treatment market during the forecast period, due to rapid healthcare infrastructure development, increasing diagnosis rates of NF1, and growing access to emerging therapies.
- Chemotherapy is expected to lead the treatment segment with a market share of 34.5%, due to its clinical effectiveness, growing physician preference, and expanding use of MEK inhibitors such as selumetinib. Despite ongoing research into alternative treatment strategies, targeted therapies currently offer the most promising outcomes with fewer complications compared to surgical options, particularly for inoperable or deeply seated plexiform neurofibromas
Report Scope and Plexiform Neurofibromas Treatment Market Segmentation
|
Attributes |
Plexiform Neurofibromas Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Plexiform Neurofibromas Treatment Market Trends
“Increasing Adoption of MEK Inhibitors as a Standard Therapeutic Approach”
- One prominent trend in Plexiform Neurofibromas Treatment is the growing adoption of targeted therapies, particularly MEK inhibitors, for managing inoperable or complex tumors.
- The chronic and progressive nature of plexiform neurofibromas, often seen in patients with neurofibromatosis type 1 (NF1), has driven demand for non-surgical and precision-based treatment options that can shrink tumors, alleviate symptoms, and improve quality of life
- For instance, the approval of selumetinib (Koselugo) by AstraZeneca and Merck & Co. marked a breakthrough as the first FDA-approved treatment specifically indicated for children with symptomatic, inoperable plexiform neurofibromas. Clinical trials have shown significant tumor shrinkage and improved function in affected patients.
- This trend is significantly transforming the therapeutic landscape for plexiform neurofibromas, offering alternatives to invasive surgery, reducing treatment-associated risks, and enabling earlier medical intervention in pediatric populations.
- The Plexiform Neurofibromas Treatment market is poised for sustained growth, driven by continued clinical research, regulatory support for orphan drugs, and increasing access to innovative therapies. As awareness of NF1 rises and healthcare systems prioritize rare disease management, targeted treatments like MEK inhibitors will remain central to advancing patient outcomes.
Plexiform Neurofibromas Treatment Market Dynamics
Driver
“Rising Prevalence of Neurofibromatosis Type 1 (NF1) and Associated Morbidity”
- The rising prevalence of Neurofibromatosis Type 1 (NF1) and its associated complications are significantly driving the demand for effective treatment options for plexiform neurofibromas.
- NF1 is one of the most common genetic disorders, affecting approximately 1 in 3,000 individuals worldwide. A significant portion of NF1 patients develop plexiform neurofibromas—benign but potentially disfiguring and debilitating tumors that can cause pain, neurological issues, and functional impairments.
- The progressive nature of these tumors, particularly in pediatric populations, necessitates early diagnosis and the availability of targeted, minimally invasive, and patient-specific treatment strategies to improve long-term outcomes and quality of life.
- As healthcare systems become more aware of rare diseases, and diagnostic capabilities improve, more cases of NF1 and associated plexiform neurofibromas are being identified at earlier stages—further emphasizing the urgency for specialized therapeutic interventions.
- The increasing recognition of NF1 as a lifelong condition with high healthcare needs has led to greater investments in research, drug development, and clinical care models focused on multidisciplinary treatment approaches.
For instance,
- The approval of Koselugo (selumetinib) by the U.S. FDA for children aged two years and older with symptomatic, inoperable plexiform neurofibromas marked a critical milestone. It demonstrated a significant reduction in tumor size in clinical trials, offering the first pharmacologic treatment specifically for this condition and validating the medical need for more targeted therapies.
- As more cases are diagnosed and awareness increases, the Plexiform Neurofibromas Treatment market is expected to experience sustained growth, driven by rising disease burden, increasing patient advocacy, and growing access to specialized care and novel treatment options.
Opportunity
“Expansion of Research and Development in Rare Disease Therapeutics”
- The expansion of research and development in rare disease therapeutics is unlocking significant growth opportunities in the Plexiform Neurofibromas Treatment market. As pharmaceutical companies and research institutions intensify their focus on orphan diseases, new avenues for innovation, funding, and drug approvals are emerging.
- This surge in R&D activity is fostering the development of novel targeted therapies, gene-based interventions, and more personalized treatment strategies for patients with neurofibromatosis type 1 (NF1), particularly those suffering from inoperable plexiform neurofibromas.
- Government incentives such as Orphan Drug Designation (ODD), Fast Track status, and Rare Pediatric Disease Priority Review Vouchers are accelerating clinical trials and regulatory pathways, encouraging increased investment in this previously underserved segment.
For instance,
- In 2020, the U.S. FDA approved Koselugo (selumetinib), a MEK inhibitor co-developed by AstraZeneca and Merck & Co., as the first drug specifically indicated for children with symptomatic, inoperable plexiform neurofibromas. This approval marked a major milestone in rare disease therapeutics.
- The continued momentum in rare disease R&D—supported by regulatory incentives and strategic collaborations—presents a substantial opportunity for market growth. As more companies prioritize the development of advanced treatments for plexiform neurofibromas, patients are expected to benefit from increased therapeutic options, improved outcomes, and enhanced quality of life.
Restraint/Challenge
“High Treatment Costs and Limited Access to Specialized Therapies in Low-Resource Settings”
- The high cost of Plexiform Neurofibromas Treatment therapies presents a significant challenge for market growth, especially in low- and middle-income countries. Many of the advanced treatments, such as MEK inhibitors and targeted biologics, come with substantial price tags, limiting their accessibility.
- These specialized therapies, often classified as orphan drugs, can cost tens to hundreds of thousands of dollars annually per patient. Without adequate insurance coverage or government subsidies, patients in resource-limited regions struggle to afford these life-altering treatments.
- The financial burden extends beyond the drugs themselves—genetic testing, advanced imaging, and multidisciplinary care associated with treatment further increase overall costs, placing strain on both patients and healthcare systems.
For instance,
- Selumetinib (Koselugo), approved in 2020 for treating inoperable plexiform neurofibromas, has an estimated annual treatment cost of over $100,000 in the U.S. This price point poses a major barrier in countries where public healthcare systems or private insurers may not fully cover rare disease treatments.
- As a result, high treatment costs contribute to significant disparities in access to care, particularly in underserved regions. This financial limitation acts as a key restraint for the Plexiform Neurofibromas Treatment market, impeding the widespread adoption of innovative therapies and delaying effective management for many patients
Plexiform Neurofibromas Treatment Market Scope
The market is segmented on the basis of type, biopsy techniques, end user, and usage.
|
Segmentation |
Sub-Segmentation |
|
By Therapy Type |
|
|
By Treatment |
|
|
By Route of Administration |
|
|
By End User |
|
In 2025, the Chemotherapy is projected to dominate the market with a largest share in therapy type segment
The Chemotherapy segment is expected to dominate the Plexiform Neurofibromas Treatment market with the largest share of 34.5% in 2025 due to its established role in managing complex and inoperable tumors, especially when surgical intervention is not feasible. Chemotherapy remains widely adopted due to its cost-effectiveness and the familiarity of oncologists with its use in multi-drug regimens. Despite the growing interest in newer treatment approaches such as targeted therapies and MEK inhibitors, chemotherapy continues to hold a central position in clinical practice, particularly in regions where access to advanced therapeutics is limited. Its broad application, availability, and integration into standard treatment protocols contribute to its sustained dominance in the market.
The Medication is expected to account for the largest share during the forecast period in treatment segment
In 2025, the Medication segment is expected to dominate the market due to its non-invasive nature, improved patient tolerance, and increasing adoption of targeted therapies such as MEK inhibitors. Unlike surgical approaches, medication-based treatments offer a systemic, radiation-free alternative that reduces the risks associated with complex tumor resections. These therapies also allow for outpatient management and more flexible treatment planning, which enhances both patient quality of life and healthcare efficiency. The growing demand for less invasive, more personalized treatment options—combined with supportive regulatory pathways and ongoing clinical trials—is further accelerating the expansion of this segment.
Plexiform Neurofibromas Treatment Market Regional Analysis
“North America Holds the Largest Share in the Plexiform Neurofibromas Treatment Market”
- North America dominates the Plexiform Neurofibromas Treatment market with a share of 36.4%, driven by advanced healthcare infrastructure, high adoption of innovative treatment options, and the presence of leading pharmaceutical and biotechnological companies.
- The U.S. holds a significant share of 78.3% due to the growing demand for targeted therapies, such as MEK inhibitors, driven by the increasing prevalence of neurofibromatosis type 1 (NF1) and the need for more effective, non-invasive treatment solutions.
- The presence of key market players like AstraZeneca, Merck & Co., and SpringWorks Therapeutics, combined with ongoing research and regulatory support for rare disease treatments, continues to drive innovation in the development of treatments for plexiform neurofibromas. The high level of investment in research and development (R&D) ensures continuous advancements in treatment options
- The increasing recognition of NF1 and its associated complications, along with rising patient awareness and the growing adoption of personalized medicine, supports the market's expansion in North America. As healthcare standards improve and more treatments become available, demand for advanced, targeted therapies will continue to rise, reinforcing North America’s dominant position in the market.
“Asia-Pacific is Projected to Register the Highest CAGR in the Plexiform Neurofibromas Treatment Market”
- The Asia-Pacific region is expected to witness the highest growth rate in the Plexiform Neurofibromas Treatment market, driven by rapid advancements in healthcare infrastructure, increasing awareness of neurofibromatosis type 1 (NF1), and a rising demand for advanced treatment options.
- Countries such as China, India, and Japan are emerging as key markets for Plexiform Neurofibromas Treatment due to the rising prevalence of NF1 and the growing need for targeted therapies. As awareness about rare diseases increases and early diagnosis initiatives gain traction, the demand for innovative and effective treatment solutions in these regions is rapidly growing.
- Japan, known for its highly developed medical infrastructure, plays a pivotal role in the adoption of advanced therapies for NF1-related tumors, including the use of MEK inhibitors and gene-based treatments. The country remains at the forefront of adopting novel, precision-based treatment methods, improving patient outcomes and treatment accuracy.
- The expanding focus on healthcare development and the rising demand for effective treatment for NF1-related complications are expected to further propel the adoption of cutting-edge treatments, positioning the APAC region as the fastest-growing market for Plexiform Neurofibromas Treatment
Plexiform Neurofibromas Treatment Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Novartis AG (Switzerland)
- AstraZeneca (United Kingdom)
- Pfizer Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- BioXcel Therapeutics, Inc. (U.S.)
- SpringWorks Therapeutics (U.S.)
- Recursion (U.S.)
- Celldex Therapeutics (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Takeda Pharmaceutical Company Limited (Japan)
- Mylan N.V. (U.S.) (Note: Mylan merged with Upjohn to form Viatris, headquartered in the U.S.)
- PTC Therapeutics (U.S.
- Noveome Biotherapeutics, Inc. (U.S.
- Sangamo Therapeutics (U.S.)
- GlaxoSmithKline plc (United Kingdom
- Eli Lilly and Company (U.S.
- Bristol-Myers Squibb Company (U.S.)
- Sanofi (France)
Latest Developments in Global Plexiform Neurofibromas Treatment Market
- In February 2025, the FDA approved mirdametinib (Gomekli) for adults and pediatric patients aged 2 and older with neurofibromatosis type 1 (NF1) who have symptomatic plexiform neurofibromas (PN) that cannot be fully resected
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.