Global Pneumocystis Carinii Pneumonia Pcp Market
Market Size in USD Million
CAGR :
% 
 USD
150.05 Million
USD
201.43 Million
2024
2032
USD
150.05 Million
USD
201.43 Million
2024
2032
| 2025 –2032 | |
| USD 150.05 Million | |
| USD 201.43 Million | |
|
|
|
|
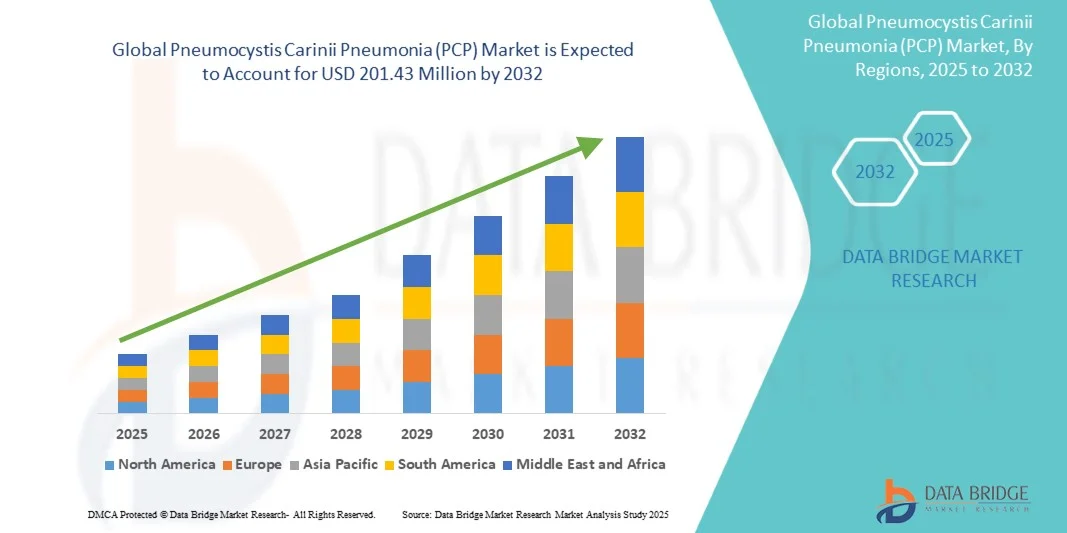
Pneumocystis Carinii Pneumonia (PCP) Market Size
- The global Pneumocystis Carinii Pneumonia (PCP) market size was valued at USD 150.05 million in 2024 and is expected to reach USD 201.43 million by 2032, at a CAGR of 3.75% during the forecast period
- The market growth is largely fueled by the rising prevalence of HIV/AIDS and immunocompromised populations, which increases susceptibility to opportunistic infections such as PCP
- Furthermore, advancements in diagnostic tools and antifungal therapies, along with increasing awareness among healthcare providers and patients, are driving early detection and effective treatment. These converging factors are accelerating the uptake of PCP therapies, thereby significantly boosting the industry’s growth
Pneumocystis Carinii Pneumonia (PCP) Market Analysis
- PCP, caused by the opportunistic fungus Pneumocystis jirovecii, primarily affects immunocompromised individuals, including patients with HIV/AIDS, organ transplant recipients, and those on immunosuppressive therapies. The market is increasingly driven by the need for effective prophylactic and therapeutic interventions to reduce morbidity and mortality in these vulnerable populations
- The escalating demand for PCP treatments is primarily fueled by the rising prevalence of HIV/AIDS globally, growing awareness of opportunistic infections, and advancements in antifungal therapies and diagnostic tools
- North America dominated the Pneumocystis Carinii Pneumonia (PCP) market with the largest revenue share of 43% in 2024, driven by a high prevalence of immunocompromised patients, early adoption of advanced healthcare solutions, and strong presence of key pharmaceutical companies focusing on innovative treatment regimens
- Asia-Pacific is expected to be the fastest-growing region in the Pneumocystis Carinii Pneumonia (PCP) market during the forecast period due to increasing HIV/AIDS awareness, improving healthcare infrastructure, and rising access to essential antifungal therapies
- Bactrim dominated the Pneumocystis Carinii Pneumonia (PCP) market with a share of 39.2% in 2024, driven by its established efficacy and widespread use as the first-line therapy for both prophylaxis and treatment of PCP in high-risk populations
Report Scope and Pneumocystis Carinii Pneumonia (PCP) Market Segmentation
|
Attributes |
Pneumocystis Carinii Pneumonia (PCP) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Pneumocystis Carinii Pneumonia (PCP) Market Trends
“Advancements in Antifungal Therapies and Diagnostic Tools”
- A significant and accelerating trend in the global PCP market is the development of novel antifungal therapies and improved diagnostic platforms, enhancing treatment outcomes and early disease detection
- For instance, next-generation molecular assays allow clinicians to rapidly and accurately detect Pneumocystis jirovecii, enabling timely intervention and optimized therapy selection
- Advances in drug formulations, including oral and parenteral options, are improving patient adherence and tolerability while reducing adverse effects, thereby increasing the overall effectiveness of PCP management
- The integration of digital health tools and electronic medical records allows better monitoring of patient response, facilitating individualized treatment adjustments and improved clinical outcomes
- This trend towards more targeted, effective, and patient-friendly treatments is reshaping clinical expectations for PCP management. Consequently, companies are investing in research for combination therapies and safer long-term prophylactic options
- The demand for more advanced antifungal drugs and rapid diagnostics is growing rapidly across hospitals and clinics, as healthcare providers increasingly prioritize efficacy, patient safety, and reduced hospitalization rates
- Development of long-acting prophylactic formulations is gaining momentum, reducing dosing frequency and improving patient compliance in high-risk populations
- Integration of AI-driven predictive analytics in treatment planning is enabling earlier identification of high-risk patients and more precise therapy selection, enhancing clinical outcomes
Pneumocystis Carinii Pneumonia (PCP) Market Dynamics
Driver
“Rising Prevalence of HIV/AIDS and Immunocompromised Populations”
- The increasing prevalence of HIV/AIDS and other immunocompromised conditions is a significant driver for heightened demand for PCP therapies
- For instance, in 2024, hospitals reported increased cases of opportunistic infections among transplant recipients, driving the adoption of prophylactic and therapeutic interventions for PCP
- As awareness of opportunistic infections grows, PCP treatments are increasingly prescribed as standard care to prevent morbidity and mortality in high-risk populations
- Furthermore, the expansion of healthcare infrastructure and improved access to essential antifungal therapies is boosting PCP treatment uptake in both developed and emerging markets
- The convenience of oral and parenteral formulations, coupled with hospital-based monitoring, is facilitating broader adoption of PCP treatments across clinics and inpatient facilities
- Increasing government initiatives and funding for infectious disease management programs are further stimulating PCP treatment demand
- Partnerships between pharmaceutical companies and healthcare providers to improve drug distribution and awareness are enhancing treatment accessibility and market penetration
Restraint/Challenge
“Drug Side Effects and Limited Awareness in Emerging Regions”
- Concerns surrounding adverse effects of standard PCP therapies, such as rashes, hematologic toxicity, or gastrointestinal disturbances, pose a challenge to treatment adherence
- For instance, reports of severe side effects in immunocompromised patients have caused clinicians to carefully monitor dosing and consider alternative therapies, which can slow widespread adoption
- Addressing these concerns through safer drug formulations, combination therapy options, and physician education is crucial for building confidence in PCP management
- In addition, limited awareness of PCP risk and prophylaxis guidelines in emerging regions restricts early diagnosis and treatment, hindering market growth
- Overcoming these challenges through awareness campaigns, training programs for healthcare providers, and development of patient-friendly treatment options will be vital for sustained market expansion
- The high cost of certain antifungal therapies remains a barrier, especially in low-income regions, limiting widespread adoption and adherence
- Regulatory hurdles for approval of new or combination therapies in multiple regions can delay market entry and slow the introduction of innovative treatment options
Pneumocystis Carinii Pneumonia (PCP) Market Scope
The market is segmented on the basis of treatment, route of administration, end-users, and distribution channel.
- By Treatment
On the basis of treatment, the Pneumocystis Carinii Pneumonia (PCP) market is segmented into bactrim, septra, proloprim, dapsone, cleocin, primaquine, mepron, pentamidine, leucovorin, and others. The Bactrim segment dominated the market with the largest revenue share of 39.2% in 2024, driven by its established efficacy and widespread adoption as the first-line therapy for both prophylaxis and treatment of PCP. Healthcare providers prefer Bactrim due to its proven effectiveness, favorable safety profile, and multiple formulation options including oral and intravenous administration. The segment’s dominance is further supported by strong physician awareness, inclusion in clinical guidelines, and high availability across hospital and retail pharmacies. Bactrim’s long-standing presence in the market and cost-effectiveness relative to newer therapies also contribute to its leading position. Its continued integration into prophylactic regimens for high-risk patient populations ensures sustained demand. The segment benefits from consistent supply chains and broad patient acceptance, making it the preferred choice for clinicians worldwide.
The Mepron segment is expected to witness the fastest growth from 2025 to 2032, fueled by increasing adoption for patients intolerant to first-line therapies and those requiring specialized treatment. Mepron (atovaquone) is preferred for its favorable side-effect profile and oral administration, making it suitable for outpatient care. The segment benefits from growing physician familiarity, expanding availability, and inclusion in updated treatment guidelines for immunocompromised populations. Rising patient preference for well-tolerated therapies, especially among transplant recipients and HIV-positive individuals, further accelerates its growth. Increasing awareness of alternative treatment options and marketing efforts by pharmaceutical companies also support adoption. The segment’s expansion is strengthened by affordability improvements and patient education programs promoting adherence.
- By Route of Administration
On the basis of route of administration, the Pneumocystis Carinii Pneumonia (PCP) market is segmented into oral, parenteral, and others. The oral segment dominated the market with a share of 55% in 2024, driven by patient convenience, ease of outpatient treatment, and higher adherence rates compared to parenteral therapy. Oral formulations allow long-term prophylactic administration, which is critical for high-risk groups such as HIV/AIDS patients and organ transplant recipients. Hospitals and clinics prefer oral options for both prophylaxis and treatment due to simplified administration and reduced need for inpatient monitoring. The availability of oral therapies in multiple dosages and fixed-dose combinations enhances their usability in various patient populations. The segment’s dominance is supported by healthcare guidelines recommending oral therapy as the primary mode of administration for most non-severe PCP cases. Physicians and pharmacists emphasize oral therapy for its safety, ease of distribution, and reduced hospital resource utilization.
The parenteral segment is expected to witness the fastest growth from 2025 to 2032, driven by increasing use in severe cases requiring hospital-based care and rapid therapeutic action. Parenteral administration ensures immediate bioavailability and is often preferred in immunocompromised patients with gastrointestinal absorption issues. Advancements in intravenous formulations, improved safety protocols, and growing hospital infrastructure in emerging markets support this growth. The segment also benefits from clinician preference for IV therapy in critical care settings, particularly among transplant units and intensive care wards. Expansion of hospital-based PCP treatment programs and adoption of newer parenteral antifungals further drive market growth. In addition, physician education and guideline updates recommending IV therapy in severe cases boost adoption rates.
- By End-Users
On the basis of end-users, the Pneumocystis Carinii Pneumonia (PCP) market is segmented into clinics, hospitals, and others. The hospitals segment dominated the market with a 48% share in 2024, reflecting the central role of inpatient care and specialized treatment facilities for immunocompromised patients. Hospitals serve as the primary point of care for both prophylactic and therapeutic administration of PCP treatments. The segment benefits from higher availability of trained medical staff, advanced diagnostic tools, and established treatment protocols. Hospitals also maintain direct procurement channels through pharmacy departments, ensuring timely access to essential therapies. The dominance of this segment is reinforced by the higher concentration of high-risk patients requiring continuous monitoring and treatment adjustments. Multidisciplinary care teams in hospitals facilitate proper dosing, adverse effect management, and follow-up, further boosting hospital demand. Availability of hospital pharmacies and integration with patient management systems strengthens its position as the primary end-user segment.
The clinics segment is expected to witness the fastest growth from 2025 to 2032, driven by expanding outpatient care services, early diagnosis initiatives, and rising patient preference for community-based treatment. Clinics are increasingly equipped with diagnostic tools and access to oral prophylactic therapies, supporting effective management in non-hospital settings. The segment growth is fueled by telemedicine integration, patient convenience, and cost-effective outpatient treatment options. Partnerships with local healthcare providers and NGOs enhance accessibility in emerging regions. Increased awareness of prophylactic care and outpatient treatment programs also contributes to rapid adoption.
- By Distribution Channel
On the basis of distribution channel, the Pneumocystis Carinii Pneumonia (PCP) market is segmented into direct tender, hospital pharmacy, retail pharmacy, online pharmacy, and others. The hospital pharmacy segment dominated the market with a 46% share in 2024, driven by direct procurement by healthcare institutions to ensure availability of essential PCP therapies. Hospitals rely on in-house pharmacy distribution to manage inventory efficiently and provide immediate access to high-risk patients. The segment’s dominance is supported by strong supply chain networks, bulk purchasing agreements, and integration with hospital management systems. Hospital pharmacies also play a critical role in ensuring quality control, proper storage, and timely administration of both prophylactic and therapeutic drugs. The segment benefits from consistent demand from inpatient and outpatient departments, reinforcing its leading position. Availability of trained pharmacy staff and established relationships with suppliers further strengthens dominance.
The online pharmacy segment is expected to witness the fastest growth from 2025 to 2032, driven by increasing e-commerce adoption, convenience of home delivery, and growing patient awareness of online healthcare services. Online channels offer accessibility to rural and remote populations, supporting adherence to long-term prophylactic regimens. The segment also benefits from expanding telehealth integration, digital prescription services, and partnerships with logistics providers. Rising consumer preference for privacy, convenience, and flexible ordering options further accelerates the segment’s growth. Growth is also supported by mobile app integration and patient education campaigns on adherence and therapy management.
Pneumocystis Carinii Pneumonia (PCP) Market Regional Analysis
- North America dominated the Pneumocystis Carinii Pneumonia (PCP) market with the largest revenue share of 43% in 2024, driven by a high prevalence of immunocompromised patients, early adoption of advanced healthcare solutions, and strong presence of key pharmaceutical companies focusing on innovative treatment regimens
- Healthcare providers in the region prioritize early diagnosis, prophylactic treatment, and effective management of PCP, supported by well-established hospital networks, advanced diagnostic tools, and comprehensive treatment guidelines
- This strong market presence is further reinforced by high physician awareness, robust supply chains, and the availability of first-line treatments such as Bactrim and Septra, ensuring timely access to critical therapies for high-risk patients
U.S. Pneumocystis Carinii Pneumonia (PCP) Market Insight
The U.S. Pneumocystis Carinii Pneumonia (PCP) market captured the largest revenue share of 83% in 2024 within North America, driven by the high prevalence of HIV/AIDS and other immunocompromised populations requiring prophylactic and therapeutic interventions. Hospitals and clinics increasingly prioritize early diagnosis and preventive treatment of PCP, supported by advanced diagnostic tools and well-established treatment protocols. The growing awareness among physicians regarding updated clinical guidelines, along with widespread access to first-line therapies such as Bactrim and Septra, fuels market adoption. The trend of integrating outpatient care with hospital monitoring ensures better treatment adherence and reduced complications. Moreover, government initiatives and public health programs promoting opportunistic infection management are significantly contributing to market growth. The presence of key pharmaceutical players with extensive supply chains further strengthens the U.S. market.
Europe Pneumocystis Carinii Pneumonia (PCP) Market Insight
The Europe Pneumocystis Carinii Pneumonia (PCP) market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by increasing awareness of opportunistic infections and the need for preventive care in immunocompromised populations. The region’s mature healthcare systems, coupled with strong government support and funding for HIV/AIDS and transplant patient care, foster the adoption of PCP therapies. Healthcare providers are increasingly utilizing standardized treatment protocols and prophylactic regimens to improve patient outcomes. European patients also benefit from widespread access to oral and parenteral formulations of antifungal drugs. Hospitals and specialized clinics are actively implementing early detection and management strategies, contributing to market growth. The region is witnessing rising adoption in both urban and semi-urban healthcare facilities, further supporting expansion.
U.K. Pneumocystis Carinii Pneumonia (PCP) Market Insight
The U.K. Pneumocystis Carinii Pneumonia (PCP) market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by a strong focus on infectious disease management and preventive healthcare initiatives. Rising awareness of PCP risk among HIV-positive and immunocompromised patients is encouraging physicians to adopt prophylactic treatment strategies. In addition, national healthcare guidelines emphasize early diagnosis and timely therapy, increasing treatment adoption. The country’s advanced healthcare infrastructure and widespread hospital networks facilitate prompt access to essential PCP therapies. Evolving outpatient care programs and telemedicine integration are also supporting growth by improving accessibility to prophylactic and therapeutic treatments. Increasing patient awareness campaigns are expected to continue driving the market.
Germany Pneumocystis Carinii Pneumonia (PCP) Market Insight
The Germany Pneumocystis Carinii Pneumonia (PCP) market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing awareness of opportunistic infections and a growing focus on preventive care among high-risk populations. Hospitals and specialized clinics play a key role in delivering standardized treatment and prophylactic regimens. Germany’s strong healthcare infrastructure, emphasis on innovation, and adherence to strict clinical guidelines promote the adoption of both first-line and alternative therapies. Integration of electronic medical records and improved diagnostic facilities ensures timely treatment for immunocompromised patients. The availability of multiple treatment options, along with government health programs targeting HIV/AIDS and transplant patients, further drives growth. Hospitals’ proactive monitoring of treatment adherence supports the market’s expansion.
Asia-Pacific Pneumocystis Carinii Pneumonia (PCP) Market Insight
The Asia-Pacific Pneumocystis Carinii Pneumonia (PCP) market is poised to grow at the fastest CAGR of 23% during 2025–2032, driven by improving healthcare infrastructure, rising awareness of HIV/AIDS and opportunistic infections, and expanding access to antifungal therapies. Countries such as China, India, and Japan are witnessing increasing investment in diagnostic tools, prophylactic programs, and hospital-based treatment initiatives. Government health initiatives and international partnerships are promoting early detection and preventive care. The growing middle-class population, rising disposable incomes, and urbanization are enabling better access to both oral and parenteral PCP therapies. Hospitals and clinics in the region are increasingly implementing standardized treatment protocols, supporting market growth. Pharmaceutical companies are also expanding their presence and distribution networks across APAC, enhancing accessibility and affordability.
Japan Pneumocystis Carinii Pneumonia (PCP) Market Insight
The Japan Pneumocystis Carinii Pneumonia (PCP) market is gaining momentum due to a high prevalence of immunocompromised patients, advanced healthcare infrastructure, and strong public health programs promoting early diagnosis and treatment. Japanese hospitals and clinics focus on both prophylactic and therapeutic interventions, ensuring timely care for transplant recipients and HIV-positive patients. Integration of advanced diagnostic technologies, such as PCR-based assays, enables rapid and accurate detection of PCP. The country’s aging population and increasing incidence of immunosuppressive therapies are further boosting demand for safe and effective treatment options. Proactive physician education and adherence to clinical guidelines support higher adoption rates. Collaborative efforts between healthcare providers and pharmaceutical companies facilitate the distribution and availability of essential PCP therapies.
India Pneumocystis Carinii Pneumonia (PCP) Market Insight
The India PCP market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to the high prevalence of HIV/AIDS, expanding healthcare infrastructure, and growing awareness of opportunistic infections. Hospitals and clinics are increasingly implementing prophylactic and therapeutic regimens for high-risk populations. The government’s initiatives to strengthen infectious disease management, combined with rising patient awareness, are driving demand for both oral and parenteral therapies. Rapid urbanization and increasing access to affordable antifungal treatments are key growth drivers. The presence of domestic pharmaceutical manufacturers ensures supply consistency and cost-effectiveness. In addition, expansion of outpatient care programs and telemedicine adoption supports better treatment adherence and market penetration.
Pneumocystis Carinii Pneumonia (PCP) Market Share
The Pneumocystis Carinii Pneumonia (PCP) industry is primarily led by well-established companies, including:
- Pfizer Inc. (U.S.)
- Aurobindo Pharma Limited (India)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Sun Pharmaceutical Industries Ltd. (India)
- GLENMARK PHARMACEUTICALS LTD. (India)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Bristol-Myers Squibb Company (U.S.)
- Merck & Co., Inc. (U.S.
- Novartis AG (Switzerland)
- AbbVie Inc. (U.S.)
- AstraZeneca (U.K.)
- Johnson & Johnson and its affiliates (U.S.)
- Sanofi (France)
- GSK plc (U.K.)
- Boehringer Ingelheim International GmbH (Germany)
What are the Recent Developments in Global Pneumocystis Carinii Pneumonia (PCP) Market?
- In September 2025, The National Institutes of Health (NIH) updated its guidelines on the prophylactic treatment of Pneumocystis jirovecii pneumonia (PCP). The updated guidelines emphasize the importance of prophylaxis in high-risk populations and provide recommendations for the use of trimethoprim-sulfamethoxazole (TMP-SMX) and alternative therapies
- In February 2025, A study was published investigating the efficacy and safety of a low-dose regimen of trimethoprim-sulfamethoxazole (TMP-SMX) for prophylaxis against Pneumocystis jirovecii pneumonia (PJP) in patients without HIV infection. The study aimed to determine if a lower dose could reduce adverse events while maintaining effectiveness
- In October 2023, a clinical trial named CaspoNEB was initiated to evaluate the efficacy and safety of daily aerosolized caspofungin in combination with conventional systemic antifungal therapy for treating Pneumocystis jirovecii pneumonia (PCP) in immunocompromised patients. This trial aims to explore alternative treatment options for PCP, especially in patients who may not tolerate standard therapies
- In May 2023, A study was published discussing the potential benefits of combination therapy for treating PCP in non-HIV-infected patients. The study suggests that combining trimethoprim-sulfamethoxazole (TMP-SMX) with an echinocandin may improve overall survival rates, particularly in moderate to severe cases of PCP
- In January 2022, A review article was published discussing advances in non-invasive diagnostic methods for Pneumocystis jirovecii pneumonia (PCP). The article highlights promising minimally invasive tests that could reduce the need for invasive respiratory sampling, potentially improving patient comfort and diagnostic efficiency
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.