Global Point Of Care Biopsy Market
Market Size in USD Billion
CAGR :
% 
 USD
3.33 Billion
USD
6.54 Billion
2024
2032
USD
3.33 Billion
USD
6.54 Billion
2024
2032
| 2025 –2032 | |
| USD 3.33 Billion | |
| USD 6.54 Billion | |
|
|
|
|
Point-of-Care Biopsy Market Size
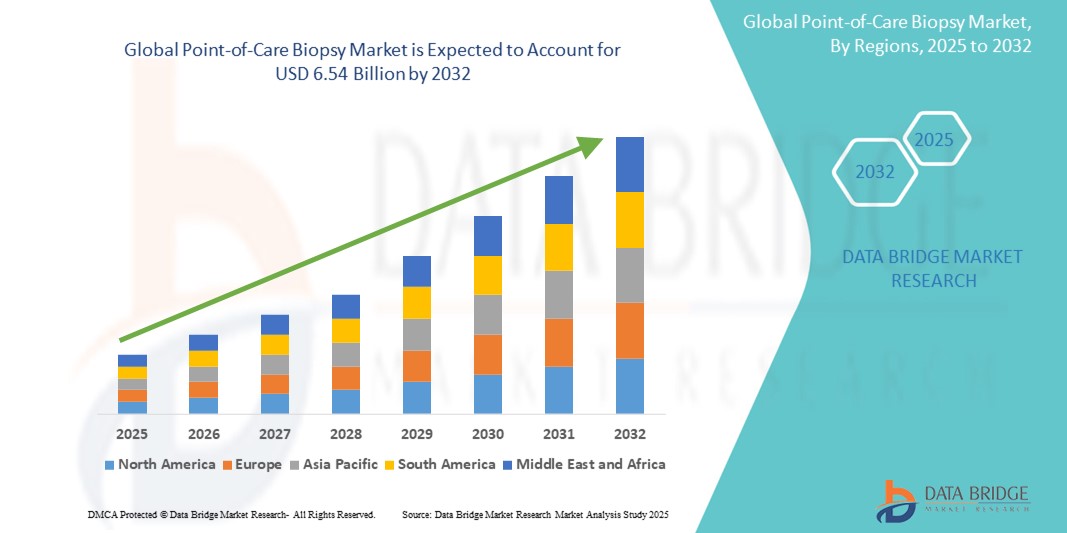
- The global point-of-care biopsy market size was valued at USD 3.33 billion in 2024 and is expected to reach USD 6.54 billion by 2032, at a CAGR of 14.50% during the forecast period
- The market growth is largely fueled by the increasing prevalence of cancer and the urgent need for rapid, accurate diagnostic tools at the patient’s bedside, driving the demand for point-of-care biopsy solutions across healthcare facilities globally
- Furthermore, advancements in minimally invasive biopsy technologies and portable diagnostic tools are establishing point-of-care biopsy as a crucial component in early cancer detection and disease management. These converging factors are accelerating the adoption of Point-of-Care Biopsy solutions, thereby significantly boosting the industry's growth
Point-of-Care Biopsy Market Analysis
- Point-of-care biopsy solutions, which allow for immediate tissue sampling and diagnostic analysis at or near the site of patient care, are becoming increasingly vital in modern clinical workflows due to their ability to provide rapid results, reduce patient anxiety, and enable timely treatment decisions in both hospital and outpatient settings
- The escalating demand for point-of-care biopsy is primarily fueled by the rising global incidence of cancer, increasing emphasis on early diagnosis, and technological advancements in portable, minimally invasive biopsy devices
- North America dominates the point-of-care biopsy market with the largest revenue share of 43.4% in 2024, characterized by advanced healthcare infrastructure, high healthcare spending, and a strong presence of leading medical device manufacturers
- Asia-Pacific is expected to be the fastest growing region in the point-of-care biopsy market during the forecast period, with a CAGR of 15.4%, due to increasing cancer awareness, expanding healthcare access in rural areas, and rising disposable incomes, particularly in countries such as China, India, and Japan
- Reagents and kits segment dominates the point-of-care biopsy market with a market share of 61.3% in 2024, driven by their extensive and recurring need for performing diagnostic tests, supporting diverse applications in oncology, infectious diseases, and genetic diagnostics
Report Scope and Point-of-Care Biopsy Market Segmentation
|
Attributes |
Point-of-Care Biopsy Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Point-of-Care Biopsy Market Trends
“Enhanced Convenience Through AI and Real-Time Diagnostic Integration”
- A significant and accelerating trend in the global point-of-care biopsy market is the integration of artificial intelligence (AI) and real-time diagnostic platforms into biopsy workflows. This convergence is dramatically improving diagnostic accuracy, speed, and convenience for both clinicians and patients
- For instance, AI-enabled point-of-care biopsy systems can rapidly analyze tissue samples at the bedside or in outpatient settings, offering real-time preliminary diagnostic support. This is particularly transformative in oncology, where early and accurate detection can significantly influence treatment outcomes
- AI integration enhances biopsy systems by enabling features such as image recognition for abnormal cells, automated reporting, and adaptive learning algorithms that improve with continued use. For instance, some digital cytology platforms now offer automated lesion classification and risk scoring directly at the point-of-care, reducing reliance on centralized lab diagnostics
- These smart biopsy systems often pair with connected diagnostic tools, such as handheld ultrasound and cloud-based pathology platforms, providing seamless data transfer and teleconsultation capabilities. This integration allows for remote collaboration between physicians and specialists, expanding access to expert care even in underserved regions
- The growing demand for immediate diagnostic insights is reshaping expectations around biopsy procedures, particularly in ambulatory and decentralized care settings. Consequently, manufacturers such as Butterfly Network and AI-focused startups are actively developing compact, AI-powered biopsy assistive tools that can guide sample collection and improve yield accuracy
- The push toward AI-integrated, real-time point-of-care diagnostics is accelerating adoption across primary care, oncology clinics, and rural health centers, where rapid decision-making is critical and access to lab infrastructure is limited
Point-of-Care Biopsy Market Dynamics
Driver
“Growing Need for Rapid, Decentralized Diagnostics Due to Rising Cancer Incidence”
- The increasing global incidence of cancer and the demand for early, accurate diagnostics are key drivers fueling the growth of the point-of-care biopsy market. As healthcare systems aim to reduce diagnostic turnaround times, point-of-care biopsy tools are emerging as essential for delivering immediate insights and facilitating faster clinical decision-making
- For instance, in March 2024, BioVentrix Inc. introduced a portable, minimally invasive biopsy platform capable of delivering near real-time tissue analysis in outpatient settings. Such innovations underscore the growing emphasis on decentralized, patient-centric care pathways that bypass traditional laboratory bottlenecks
- Point-of-care biopsy solutions enable clinicians to obtain diagnostic tissue samples and conduct preliminary assessments on-site—significantly improving workflow efficiency in ambulatory care, oncology clinics, and resource-limited settings
- The increasing preference for less invasive, faster, and more accessible diagnostics is pushing healthcare providers and governments to integrate such technologies into standard care, particularly in regions with limited pathology infrastructure
- Moreover, technological advancements, including AI-enhanced cytology and integration with handheld imaging devices, are enhancing diagnostic accuracy and further promoting the adoption of point-of-care biopsy tools
Restraint/Challenge
“Challenges Related to Diagnostic Accuracy and Cost Constraints”
- Despite the potential of point-of-care biopsy tools, concerns regarding the consistency and accuracy of diagnostics remain a key challenge. Variability in tissue sampling techniques, lack of immediate histopathology support, and device limitations can lead to inconclusive or suboptimal results, limiting clinical confidence in certain settings
- For instance, studies have indicated that while rapid on-site evaluations (ROSE) can be highly effective, their accuracy heavily depends on the operator’s expertise and the quality of integrated imaging or cytological assessment tools
- Moreover, the relatively high cost of advanced point-of-care biopsy systems, including AI-based platforms and portable imaging devices, presents a barrier to widespread adoption—especially in developing economies and smaller healthcare facilities
- Organizations and providers with limited budgets may find it difficult to justify the initial investment without clear reimbursement pathways or demonstrable cost-benefit outcomes
- Addressing these challenges will require standardized clinical protocols, continuous user training, and broader insurance coverage or public health funding. In addition, the development of cost-effective, easy-to-use biopsy devices tailored for low-resource environments is essential for expanding access and market penetration
Point-of-Care Biopsy Market Scope
The point-of-care biopsy market is segmented into three notable segments based on product type, application, and end user.
• By Product Type
On the basis of product type, the point-of-care biopsy market is segmented into guidance systems, needles, guns, reagents and kits, and others. Reagents and kits segment dominates the market with a market share of 61.3% in 2024, driven by their extensive and recurring need for performing diagnostic tests, supporting diverse applications in oncology, infectious diseases, and genetic diagnostics.
The guidance systems segment is projected to witness the fastest CAGR of 22.1% from 2025 to 2032, propelled by rising adoption of portable ultrasound and navigation tools that enhance the precision and success rate of on-site biopsies, especially in outpatient and low-resource settings.
• By Application
On the basis of application, the market is segmented into medical diagnosis and scientific research. The medical diagnosis segment dominated with a market share of 71.5% in 2024, attributed to the increasing global cancer burden and demand for early detection tools that allow for real-time biopsy analysis near the patient care site.
The scientific research segment is expected to witness the fastest CAGR from 2025 to 2032, fueled by ongoing advancements in personalized medicine, biomarker discovery, and tissue-based drug development, requiring high-throughput biopsy capabilities in research labs.
• By End User
On the basis of end user, the point-of-care biopsy market is segmented into oncology centers, diagnostic centers, and research institutes. The oncology centers segment held the largest revenue share of 44.9% in 2024, owing to the high frequency of biopsies conducted for cancer diagnosis, staging, and treatment monitoring.
The diagnostic centers segment is expected to grow at the fastest CAGR of 20.6% from 2025 to 2032, driven by increasing decentralization of diagnostics, expansion of ambulatory care services, and adoption of rapid biopsy platforms that offer faster turnaround and enhanced patient throughput.
Point-of-Care Biopsy Market Regional Analysis
- North America dominates the point-of-care biopsy market with the largest revenue share of 43.4% in 2024, driven by the rising incidence of cancer, increasing demand for rapid diagnostic procedures, and strong presence of technologically advanced healthcare infrastructure
- Healthcare providers in the region highly value the speed, portability, and minimally invasive nature of point-of-care biopsy tools, which are essential for accelerating diagnostic workflows and improving patient outcomes, especially in oncology and outpatient settings
- This widespread adoption is further supported by favorable reimbursement frameworks, robust investment in medical innovation, and growing emphasis on personalized medicine, establishing point-of-care biopsy solutions as a critical tool in both hospital and ambulatory care environments across North America
U.S. Point-of-Care Biopsy Market Insight
The U.S. point-of-care biopsy market captured the largest revenue share of 72.3% in 2024 within North America, driven by a high prevalence of cancer, strong demand for rapid diagnostics, and widespread availability of advanced biopsy devices. The robust healthcare infrastructure, coupled with significant R&D investments and early adoption of point-of-care technologies, supports market expansion. In addition, the increasing shift towards outpatient care and minimally invasive procedures in the U.S. further accelerates the adoption of point-of-care biopsy tools across hospitals, oncology clinics, and diagnostic centers.
Europe Point-of-Care Biopsy Market Insight
The Europe point-of-care biopsy market is projected to expand at a substantial CAGR during the forecast period, fueled by stringent healthcare policies and an increased focus on early cancer detection. The demand for rapid, bedside diagnostics is rising, particularly in countries such as Germany, France, and the U.K., where aging populations and cancer incidence are growing. Technological innovation, government screening programs, and improved access to diagnostic tools are fostering adoption in both public and private healthcare sectors across the region.
U.K. Point-of-Care Biopsy Market Insight
The U.K. point-of-care biopsy market is anticipated to grow at a noteworthy CAGR during the forecast period, bolstered by a strong healthcare infrastructure and rising awareness regarding early cancer screening. National initiatives aimed at reducing diagnostic delays and improving patient outcomes are fueling demand for minimally invasive biopsy solutions. The integration of portable biopsy devices in primary care and community settings is contributing significantly to market penetration across both NHS and private healthcare sectors.
Germany Point-of-Care Biopsy Market Insight
The Germany point-of-care biopsy market is expected to expand at a CAGR from 2025 to 2032, driven by the increasing incidence of cancer and rising preference for decentralized diagnostics. Germany’s focus on precision medicine and personalized treatment protocols is encouraging adoption of real-time diagnostic tools, including biopsy kits and imaging-guided sampling devices. The presence of key medical device manufacturers and strong reimbursement support further strengthens Germany's position in the European market.
Asia-Pacific Point-of-Care Biopsy Market Insight
The Asia-Pacific point-of-care biopsy market is poised to grow at the fastest CAGR of 15.4% during the forecast period of 2025 to 2032, led by increasing cancer prevalence, growing healthcare investments, and expanding access to diagnostic services. Rapid urbanization, rising disposable incomes, and government initiatives supporting early cancer screening are accelerating demand in countries like China, India, and Japan. In addition, APAC’s role as a manufacturing hub allows for cost-effective production and broad distribution of biopsy devices, boosting affordability and accessibility across the region.
Japan Point-of-Care Biopsy Market Insight
The Japan point-of-care Biopsy market is gaining momentum due to its advanced healthcare system, rapidly aging population, and increased focus on early-stage cancer detection. The demand for non-invasive, portable diagnostic solutions is driving adoption in both clinical and homecare settings. Moreover, the integration of biopsy devices with digital imaging and AI-based diagnostic platforms is improving accuracy and efficiency, positioning Japan as a leader in precision oncology diagnostics within the APAC region.
China Point-of-Care Biopsy Market Insight
The China point-of-care biopsy market accounted for the largest market revenue share in Asia Pacific in 2024, driven by the country’s high cancer burden, improving healthcare access, and rapid medical technology adoption. China's expanding middle class and increased health awareness have led to strong demand for accessible diagnostic tools in both urban and rural areas. Supportive government policies for cancer screening programs and the proliferation of local medical device manufacturers are key contributors to the market’s robust growth.
Point-of-Care Biopsy Market Share
The point-of-care biopsy industry is primarily led by well-established companies, including:
- BD (U.S.)
- Danaher Corporation (U.S.)
- Boston Scientific Corporation (U.S.)
- Hologic, Inc. (U.S.)
- Argon Medical Devices (U.S.)
- Medtronic (Ireland)
- INRAD, Inc. (U.S.)
- Cook Medical (U.S.)
- Cardinal Health (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Qiagen (Germany)
- Johnson & Johnson Services, Inc. (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- Mammotome (U.S.)
- BioMark Diagnostics Inc. (Canada)
Latest Developments in Global Point-of-Care Biopsy Market
- In May 2024, Inocras entered into an agreement with IMBdx to enhance cancer patient care in the U.S. by integrating advanced diagnostic technologies, including whole-genome sequencing and liquid biopsy. This collaboration aims to leverage cutting-edge genomic insights for more precise cancer diagnosis and management. The initiative underscores the increasing significance of comprehensive genomic profiling in personalized oncology, contributing to more targeted and effective treatment strategies in the point-of-care biopsy market
- In May 2024, Karius, a genomic diagnostics company, successfully raised USD 100 million in a Series C funding round. This significant investment will likely fuel further research and development in their genomic diagnostic platforms, which have implications for rapid, point-of-care infectious disease detection and potentially non-invasive diagnostic approaches that complement biopsy. The funding highlights investor confidence in advanced genomic technologies for accelerating diagnostics in clinical settings
- In April 2024, NewBiologix SA, a technology innovation company, introduced a next-generation sequencing (NGS) and optical mapping platform. This innovative suite of technologies offers comprehensive genomic analysis services, directly impacting the capabilities of liquid biopsy by improving sensitivity, specificity, and scalability. Such advancements in sequencing platforms are crucial for the development of more accurate and accessible point-of-care liquid biopsy solutions, propelling the market's growth
- In April 2024, ANGLE plc (U.K.) and AstraZeneca ANGLE announced a second contract with AstraZeneca, initially valued at GBP 550,000, for the development of a circulating tumor cell (CTC)-based Androgen Receptor (AR) assay. This project commenced in June 2024 and successfully completed assay development by March 2025. This collaboration underscores the increasing integration of liquid biopsy technologies, particularly CTC analysis, into pharmaceutical research and clinical trials for targeted therapy development in prostate cancer, enhancing non-invasive monitoring capabilities
- In November 2023, Yourgene Health (a Novacyt subsidiary) and Laboriad (Morocco) Yourgene Health partnered with Laboriad to launch Morocco's first non-invasive prenatal testing (NIPT) platform. While not a "biopsy" in the traditional sense, NIPT is a form of liquid biopsy that analyzes cell-free fetal DNA from maternal blood, offering a non-invasive alternative for detecting genetic disorders. This initiative expands access to advanced prenatal screening at the point of care, demonstrating the broader adoption of non-invasive molecular diagnostic solutions
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.