Global Polymerase Chain Reaction Testing Market
Market Size in USD Billion
CAGR :
% 
 USD
9.15 Billion
USD
15.95 Billion
2024
2032
USD
9.15 Billion
USD
15.95 Billion
2024
2032
| 2025 –2032 | |
| USD 9.15 Billion | |
| USD 15.95 Billion | |
|
|
|
|
Polymerase Chain Reaction Testing Market Size
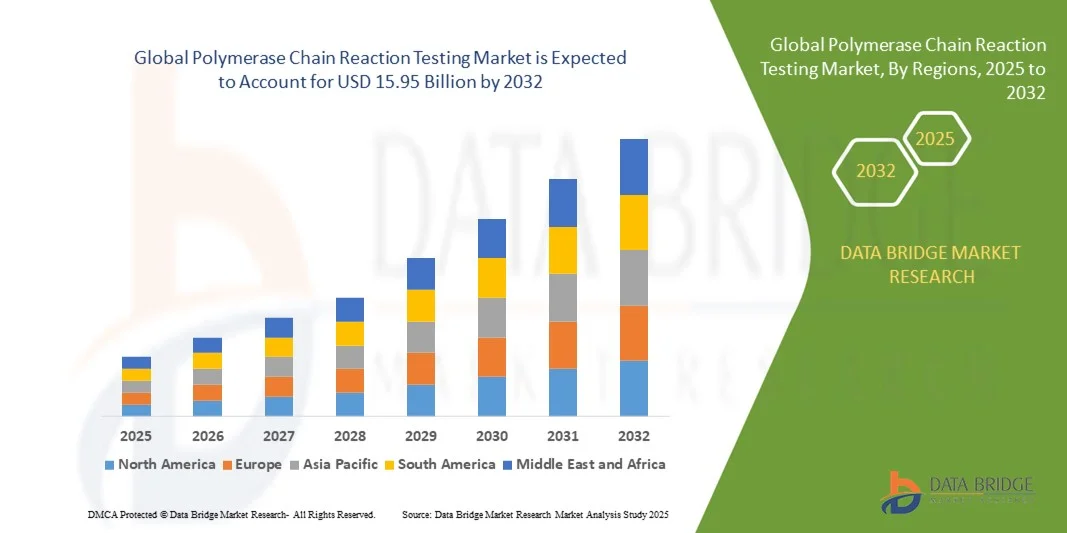
- The global polymerase chain reaction testing market size was valued at USD 9.15 billion in 2024 and is expected to reach USD 15.95 billion by 2032, at a CAGR of 7.20% during the forecast period
- The market growth is largely fuelled by the rising prevalence of infectious diseases, increasing demand for early and accurate diagnostics, and the growing adoption of molecular testing in clinical laboratories
- In addition, technological advancements such as digital PCR and real-time PCR are enhancing test sensitivity and reliability, driving greater utilization across research, clinical, and forensic applications
Polymerase Chain Reaction Testing Market Analysis
- The global PCR testing market is experiencing steady expansion owing to its crucial role in disease detection, genetic research, and personalized medicine development
- The rising demand for point-of-care molecular diagnostics and automation in laboratory workflows is further accelerating market growth, as healthcare systems emphasize rapid and precise results for patient management
- North America dominated the polymerase chain reaction testing market with the largest revenue share of 38.64% in 2024, driven by advanced healthcare infrastructure, strong R&D activities, and high demand for molecular diagnostics. The region’s emphasis on early disease detection, precision medicine, and genomic research continues to support extensive PCR testing adoption across laboratories and hospitals
- Asia-Pacific region is expected to witness the highest growth rate in the global polymerase chain reaction testing market, driven by rising demand for precision diagnostics, rapid expansion of healthcare facilities, and government initiatives supporting biotechnology development
- The biotracing products segment held the largest market share in 2024 owing to its critical role in tracking microbial and genetic contamination across various industries such as food safety, pharmaceuticals, and healthcare. Its high accuracy and ability to detect trace amounts of contaminants have made it an essential tool in maintaining product integrity and compliance with safety standards
Report Scope and Polymerase Chain Reaction Testing Market Segmentation
|
Attributes |
Polymerase Chain Reaction Testing Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
• Thermo Fisher Scientific Inc. (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Polymerase Chain Reaction Testing Market Trends
Advancements In Digital And Real-Time PCR Technologies
- The increasing shift toward digital and real-time PCR technologies is revolutionizing molecular diagnostics by offering enhanced sensitivity, precision, and quantitative accuracy. These advanced platforms enable researchers and clinicians to detect and measure low-abundance genetic targets, leading to improved diagnostic outcomes in infectious diseases and oncology applications. In addition, their ability to minimize background noise and detect rare variants makes them indispensable tools for precision medicine and genetic research
- Growing demand for rapid and reliable testing methods in clinical and research settings is driving the adoption of real-time PCR systems. Their ability to deliver fast and reproducible results supports critical decision-making in hospitals, laboratories, and public health facilities. The real-time monitoring of amplification curves also provides valuable data insights, improving the accuracy and traceability of diagnostic procedures
- Digital PCR is gaining popularity due to its superior capability in detecting rare mutations and providing absolute quantification without the need for reference standards. This technology is particularly beneficial for applications such as liquid biopsy, gene expression analysis, and pathogen detection. Furthermore, digital PCR’s ability to partition samples into thousands of micro-reactions significantly improves precision, reducing false negatives and ensuring reliable detection even in complex biological samples
- For instance, in 2024, several biotechnology companies launched next-generation PCR platforms featuring integrated data analytics and automation to enhance throughput and accuracy in molecular diagnostics workflows. These innovations have reduced turnaround times while enabling simultaneous analysis of multiple biomarkers. The integration of artificial intelligence into PCR workflows is further optimizing result interpretation, driving efficiency and diagnostic confidence
- While advanced PCR technologies are transforming diagnostic efficiency, the high cost of equipment and maintenance remains a key restraint. Continued innovation aimed at cost reduction and portability is expected to accelerate broader adoption across healthcare systems globally. Developing countries, in particular, are focusing on compact, battery-operated PCR systems to expand testing capabilities in rural and low-resource areas
Polymerase Chain Reaction Testing Market Dynamics
Driver
Rising Prevalence Of Infectious Diseases And Growing Demand For Rapid Diagnostics
- The growing incidence of infectious diseases such as COVID-19, influenza, and tuberculosis has significantly driven the need for rapid and accurate PCR testing solutions. PCR remains the gold standard for pathogen detection due to its high sensitivity and ability to identify genetic material even in low viral loads. Its role in large-scale screening and pandemic preparedness has positioned it as a vital component of modern healthcare systems
- Healthcare institutions are increasingly adopting PCR-based diagnostics for early detection, outbreak control, and surveillance purposes. The ability of PCR to detect multiple pathogens in a single assay enhances testing efficiency and patient management. This multiplexing capability reduces time and costs while improving diagnostic precision, thereby supporting faster therapeutic interventions
- Governments and global health organizations are investing heavily in strengthening diagnostic infrastructure, particularly in developing countries, to improve disease surveillance and control programs. This expansion supports greater accessibility to PCR testing services. Large-scale public health initiatives and research collaborations are further accelerating global testing capacity and preparedness
- For instance, in 2023, the World Health Organization (WHO) partnered with several diagnostic companies to distribute portable PCR testing devices across African and Southeast Asian regions to enhance early disease detection. This initiative also focused on training healthcare personnel and establishing mobile testing units to reach remote areas. As a result, several nations reported improved outbreak tracking and reduced diagnostic delays
- Although the demand for PCR testing continues to rise, ensuring affordability and accessibility across resource-limited regions remains essential. Focused efforts toward miniaturization and automation are expected to sustain growth and broaden market reach. Collaborative ventures between governments and private players are anticipated to drive innovations that balance performance with cost-efficiency
Restraint/Challenge
High Equipment Costs And Limited Accessibility In Resource-Limited Settings
- The high cost of PCR instruments, reagents, and consumables poses a major challenge to widespread adoption, especially in low- and middle-income countries. Advanced systems such as real-time and digital PCR often require significant capital investment and ongoing maintenance expenses. Consequently, smaller diagnostic centers often depend on outsourcing, which can delay diagnosis and treatment outcomes
- Many small laboratories and diagnostic centers lack the technical expertise and infrastructure required to operate and maintain complex PCR systems, leading to reliance on centralized testing facilities and delayed results. This dependence increases operational inefficiencies and limits scalability in public health systems. Training programs and simplified technologies are needed to bridge this operational skill gap
- Supply chain disruptions and limited reagent availability further impede consistent testing capacity, particularly during public health emergencies or high-demand periods. Limited access to critical consumables also impacts research projects and routine surveillance operations. Establishing local reagent manufacturing and distribution networks can help minimize such disruptions
- For instance, in 2023, several diagnostic facilities in Latin America reported delays in test processing due to shortages of PCR reagents and high import tariffs on diagnostic equipment. These challenges resulted in testing backlogs and slower disease containment responses. Governments across the region are now exploring incentives for local production to improve supply resilience
- Overcoming these challenges requires greater investment in localized manufacturing, training programs, and cost-efficient testing solutions. The development of compact, user-friendly, and affordable PCR platforms will be key to ensuring equitable access to molecular diagnostics worldwide. Partnerships between global diagnostic firms and regional healthcare providers are vital for sustainable growth and capacity building
Polymerase Chain Reaction Testing Market Scope
The market is segmented on the basis of function, application, finished food product, and type.
- By Function
On the basis of function, the polymerase chain reaction testing market is segmented into biotracing products, identifying the source of contamination, enumeration of pathogens, and sample screening. The biotracing products segment held the largest market share in 2024 owing to its critical role in tracking microbial and genetic contamination across various industries such as food safety, pharmaceuticals, and healthcare. Its high accuracy and ability to detect trace amounts of contaminants have made it an essential tool in maintaining product integrity and compliance with safety standards.
The sample screening segment is expected to witness the fastest growth rate from 2025 to 2032, driven by the increasing demand for high-throughput testing and rapid pathogen detection in food and environmental monitoring. Continuous advancements in automated PCR platforms and portable testing systems are further supporting the adoption of sample screening methods for large-scale diagnostics and surveillance applications.
- By Application
On the basis of application, the polymerase chain reaction testing market is segmented into food irrigation water, environmental samples collected in the food processing facility, and detection of genetically modified organisms. The environmental samples collected in the food processing facility segment dominated the market in 2024, supported by the rising emphasis on ensuring hygiene, contamination control, and food safety compliance across manufacturing environments. Regular environmental monitoring using PCR technology helps identify potential microbial hazards before they impact production.
The detection of genetically modified organisms (GMO) segment is projected to record the highest CAGR from 2025 to 2032, owing to the growing regulatory focus on labeling transparency and genetic authenticity in food products. PCR-based GMO detection offers superior accuracy and speed, making it a preferred method for quality assurance in agriculture and food testing laboratories.
- By Finished Food Product
On the basis of finished food product, the polymerase chain reaction testing market is segmented into fresh and processed. The processed food segment accounted for the largest revenue share in 2024, driven by the rising demand for safety verification and pathogen testing in packaged and ready-to-eat foods. Manufacturers are increasingly integrating PCR testing into routine quality control to ensure product safety and meet stringent food safety regulations.
The fresh food segment is expected to witness the fastest growth rate from 2025 to 2032 due to increasing consumer awareness regarding fresh produce safety and the risk of microbial contamination during storage and transport. The adoption of rapid PCR testing for fresh foods is gaining momentum as producers aim to enhance traceability and maintain freshness without compromising safety.
- By Type
On the basis of type, the polymerase chain reaction testing market is segmented into real-time PCR, reverse-transcriptase, multiplex PCR, nested PCR, and others. The real-time PCR segment held the largest market revenue share in 2024 due to its superior speed, accuracy, and quantitative capabilities that make it indispensable for both diagnostic and research purposes. Its ability to monitor amplification in real-time allows for faster decision-making in clinical and food testing environments.
The multiplex PCR segment is expected to witness the fastest growth from 2025 to 2032, driven by the increasing need to detect multiple pathogens simultaneously within a single reaction. This technique offers time and cost efficiency, making it ideal for large-scale testing in public health laboratories, environmental testing, and agricultural biotechnology applications.
Polymerase Chain Reaction Testing Market Regional Analysis
- North America dominated the polymerase chain reaction testing market with the largest revenue share of 38.64% in 2024, driven by advanced healthcare infrastructure, strong R&D activities, and high demand for molecular diagnostics. The region’s emphasis on early disease detection, precision medicine, and genomic research continues to support extensive PCR testing adoption across laboratories and hospitals
- The growing prevalence of infectious and chronic diseases, coupled with government funding for molecular diagnostic research, has further boosted the market. The availability of advanced real-time and digital PCR systems has strengthened North America’s leadership in the global landscape, ensuring faster and more accurate diagnostic outcomes
- High awareness levels, rapid technological advancements, and the presence of leading diagnostic companies contribute to the market’s robust position, establishing North America as a hub for PCR testing innovation and commercialization
U.S. Polymerase Chain Reaction Testing Market Insight
The U.S. polymerase chain reaction testing market captured the largest revenue share in 2024 within North America, driven by the country’s advanced healthcare infrastructure and high diagnostic testing rates. The widespread use of PCR in infectious disease detection, genetic testing, and oncology has propelled its dominance. Continuous innovation in PCR instruments and automation is enhancing workflow efficiency and accuracy in laboratories. Furthermore, the growing adoption of point-of-care PCR devices and FDA approvals for novel diagnostic assays continue to strengthen the market outlook in the U.S.
Europe Polymerase Chain Reaction Testing Market Insight
The Europe polymerase chain reaction testing market is expected to witness steady growth from 2025 to 2032, fuelled by increasing government initiatives for disease surveillance and advancements in healthcare infrastructure. Rising awareness about early disease diagnosis and strong adoption of PCR-based technologies in research and clinical applications are accelerating market expansion. The region is also witnessing a surge in molecular diagnostic collaborations, fostering accessibility and affordability of PCR testing across key markets.
U.K. Polymerase Chain Reaction Testing Market Insight
The U.K. polymerase chain reaction testing market is expected to witness the fastest growth rate from 2025 to 2032, supported by rising investments in molecular diagnostics and growing research in genomics. The country’s strong academic and clinical research ecosystem encourages the use of PCR for pathogen detection and genetic studies. Moreover, the integration of PCR testing in national health programs for infectious disease surveillance continues to enhance diagnostic efficiency and improve public health outcomes.
Germany Polymerase Chain Reaction Testing Market Insight
The Germany polymerase chain reaction testing market is projected to experience significant growth during 2025–2032, driven by the country’s focus on technological innovation and precision diagnostics. Germany’s well-established biotech and pharmaceutical sectors are heavily investing in PCR-based R&D for applications in oncology and infectious diseases. The rising adoption of automated PCR systems in diagnostic laboratories is improving turnaround times and reliability, further strengthening the market’s position in Europe.
Asia-Pacific Polymerase Chain Reaction Testing Market Insight
The Asia-Pacific polymerase chain reaction testing market is expected to witness the fastest growth rate from 2025 to 2032, attributed to expanding healthcare infrastructure, increased awareness about molecular diagnostics, and rising disease prevalence. Countries such as China, Japan, and India are heavily investing in diagnostic laboratories and PCR-based technologies to enhance healthcare accessibility. Growing government support for pandemic preparedness and infectious disease surveillance is further stimulating regional market growth.
Japan Polymerase Chain Reaction Testing Market Insight
The Japan polymerase chain reaction testing market is expected to witness significant growth from 2025 to 2032 due to its strong technological base and focus on diagnostic innovation. The country’s healthcare system is increasingly integrating PCR testing for precision medicine and infectious disease control. Japan’s biotechnology and academic sectors are actively developing miniaturized and rapid PCR solutions to support on-site diagnostics, further expanding the market’s reach across clinical and research settings.
China Polymerase Chain Reaction Testing Market Insight
The China polymerase chain reaction testing market accounted for the largest revenue share in Asia-Pacific in 2024, driven by strong government support for healthcare modernization and large-scale disease testing programs. The rapid establishment of molecular diagnostic facilities, coupled with a thriving biotechnology sector, is propelling PCR adoption across hospitals, research centers, and laboratories. China’s expanding population and emphasis on early disease detection continue to reinforce its position as a key growth driver in the global PCR testing market.
Polymerase Chain Reaction Testing Market Share
The Polymerase Chain Reaction Testing industry is primarily led by well-established companies, including:
• Thermo Fisher Scientific Inc. (U.S.)
• Agilent Technologies, Inc. (U.S.)
• Merck KGaA (Germany)
• Abbott Laboratories (U.S.)
• Beckman Coulter, Inc. (U.S.)
• Bio-Rad Laboratories, Inc. (U.S.)
• QIAGEN (Germany)
• BD (U.S.)
• F. Hoffmann-La Roche Ltd. (Switzerland)
• bioMérieux SA (France)
• Danaher (U.S.)
• LGC Limited (U.K.)
• Fluidigm Corporation (U.S.)
• Takara Bio Inc. (Japan)
• Shimadzu Corporation (Japan)
• Elsevier Inc. (U.K.)
• Cepheid (U.S.)
• PerkinElmer Inc. (U.S.)
• Eppendorf AG (Germany)
• Fluidigm Corporation (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Global Polymerase Chain Reaction Testing Market, Supply Chain Analysis and Ecosystem Framework
To support market growth and help clients navigate the impact of geopolitical shifts, DBMR has integrated in-depth supply chain analysis into its Global Polymerase Chain Reaction Testing Market research reports. This addition empowers clients to respond effectively to global changes affecting their industries. The supply chain analysis section includes detailed insights such as Global Polymerase Chain Reaction Testing Market consumption and production by country, price trend analysis, the impact of tariffs and geopolitical developments, and import and export trends by country and HSN code. It also highlights major suppliers with data on production capacity and company profiles, as well as key importers and exporters. In addition to research, DBMR offers specialized supply chain consulting services backed by over a decade of experience, providing solutions like supplier discovery, supplier risk assessment, price trend analysis, impact evaluation of inflation and trade route changes, and comprehensive market trend analysis.
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.