Global Portable Molecular Platforms Market
Market Size in USD Billion
CAGR :
% 
 USD
1.08 Billion
USD
2.12 Billion
2024
2032
USD
1.08 Billion
USD
2.12 Billion
2024
2032
| 2025 –2032 | |
| USD 1.08 Billion | |
| USD 2.12 Billion | |
|
|
|
|
Portable Molecular Platforms Market Size
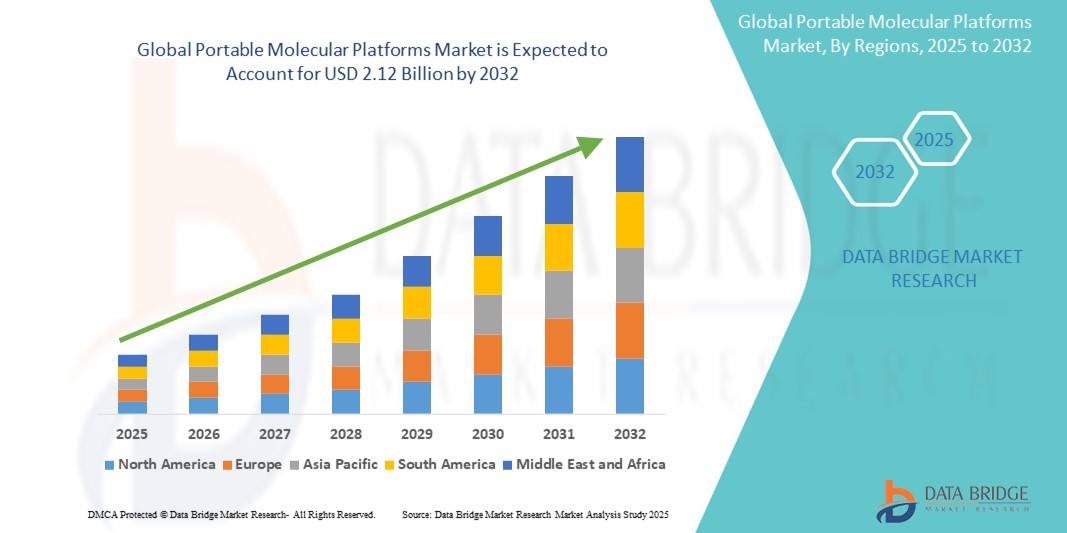
- The global portable molecular platforms market size was valued at USD 1.08 Billion in 2024 and is expected to reach USD 2.12 Billion by 2032, at a CAGR of 20.10% during the forecast period
- The market growth is largely fueled by the growing adoption and technological progress within connected healthcare devices and molecular diagnostic technologies, leading to increased digitalization in both clinical and non-clinical settings
- Furthermore, rising consumer demand for secure, user-friendly, and integrated diagnostic solutions for their health monitoring is establishing portable molecular diagnostics as the modern choice for rapid and accurate testing. These converging factors are accelerating the uptake of portable molecular diagnostic solutions, thereby significantly boosting the industry's growth
Portable Molecular Platforms Market Analysis
- Portable molecular diagnostics devices, enabling rapid and accurate detection of diseases at the point of care, are becoming essential tools in modern healthcare settings. Their integration into clinical workflows enhances patient outcomes through timely decision-making and treatment initiation
- The escalating demand for these devices is primarily fueled by the rising prevalence of infectious diseases, the need for decentralized healthcare solutions, and advancements in molecular technologies that allow for miniaturization and automation of diagnostic processes
- North America dominates the portable molecular platforms market with the largest revenue share of 46.5% in 2024, characterized by region's advanced healthcare infrastructure, high adoption rates of innovative diagnostic tools, and the presence of key industry players
- Asia-Pacific is expected to be the fastest growing region in the portable molecular platforms market during the forecast period due to increasing healthcare investments, rising awareness of early disease detection, and the expansion of healthcare services in emerging economies
- Assays & Kits segment dominates the portable molecular platforms market with a market share of 53.2% in 2024, driven by its widespread use in rapid diagnostics and ease of integration into point-of-care testing environments
Report Scope and Portable Molecular Platforms Market Segmentation
|
Attributes |
Portable Molecular Platforms Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Portable Molecular Platforms Market Trends
“Enhanced Convenience Through Integration and Automation”
- A significant and accelerating trend in the global portable molecular platforms market is the integration of advanced automation and connectivity features, enabling seamless, user-friendly operation in diverse healthcare settings
- For instance, devices such as the Cepheid GeneXpert and Abbott ID NOW incorporate automated sample processing with connectivity to electronic health records (EHR), allowing rapid test results to be easily accessed and shared by healthcare providers
- Automation in portable molecular platforms reduces human error, shortens turnaround times, and enhances test accuracy. Connectivity features enable remote monitoring, real-time data sharing, and integration into broader digital health ecosystems, improving patient management and workflow efficiency
- The growing adoption of mobile health (mHealth) applications linked with molecular diagnostic platforms allows users and clinicians to track test outcomes conveniently, promoting timely clinical decisions
- This trend toward smarter, more connected, and automated molecular diagnostic tools is reshaping expectations for point-of-care testing. Leading companies such as Roche and BioFire Diagnostics are investing in platforms with enhanced automation, cloud connectivity, and AI-powered analytics
- Demand for portable molecular platforms that combine rapid diagnostics with seamless digital integration is rising rapidly across hospitals, clinics, and home healthcare, as providers and patients increasingly prioritize convenience, accuracy, and comprehensive health monitoring
Portable Molecular Platforms Market Dynamics
Driver
“Increasing Demand Fueled by Rising Healthcare Needs and Technological Advancements”
- The growing prevalence of infectious diseases and chronic conditions worldwide, coupled with advancements in molecular diagnostic technologies, is a key driver propelling the demand for portable molecular platforms
- For instance, in early 2024, Abbott launched the updated ID NOW molecular platform with enhanced rapid testing capabilities for infectious diseases, underscoring ongoing innovation by major players expected to fuel market growth
- As healthcare providers and patients prioritize faster, accurate, and decentralized diagnostic solutions, portable molecular platforms offer rapid detection, ease of use, and reduced reliance on centralized laboratories, making them vital in diverse clinical and non-clinical settings
- Moreover, the increasing integration of these platforms into telemedicine and digital health frameworks enables remote monitoring and real-time data sharing, enhancing patient management and healthcare delivery efficiency
- The demand for portable, user-friendly, and cost-effective molecular diagnostic devices is further boosted by expanding healthcare infrastructure in emerging economies and rising consumer awareness about early disease detection
- These converging factors—including technological innovation, rising healthcare needs, and growing digital health integration—are driving accelerated adoption across hospitals, clinics, and homecare environments, significantly contributing to market growth
Restraint/Challenge
“Concerns Over Data Security and High Initial Investment Costs”
- Concerns about data security and patient privacy related to connected portable molecular platforms present a significant challenge to wider market adoption. As these devices increasingly rely on cloud connectivity and software integration, risks of cyberattacks and data breaches raise apprehensions among healthcare providers and patients
- For instance, reports of vulnerabilities in medical IoT devices have made some institutions cautious in adopting fully connected diagnostic systems without stringent security assurances
- Addressing these concerns through strong data encryption, secure user authentication, and regular software updates is critical for building trust. Companies such as Roche and BioFire emphasize their compliance with healthcare data security standards and robust cybersecurity frameworks to reassure users
- In addition, the relatively high upfront cost of advanced portable molecular platforms compared to conventional diagnostic methods can be a barrier, especially in resource-limited settings. Although costs are gradually declining with technological maturation, premium devices featuring multiplex testing and rapid turnaround times often remain expensive for smaller clinics or emerging markets
- Overcoming these challenges through enhanced cybersecurity, educating end users about secure device operation, and developing cost-effective platforms will be essential for sustained growth in the portable molecular diagnostics market
Portable Molecular Platforms Market Scope
The market is segmented on the basis of product and services, technology, application, end user, and test location.
- By Product and Services
On the basis of product & services, the market is segmented into assays & kits, instruments & analyzers, and software & services. The assays & kits segment dominates the market with a share of 53.2% in 2024, driven by their critical role in enabling rapid and accurate molecular testing across a wide range of diseases. These kits are preferred for their portability, ease of use, and ability to deliver quick results, making them essential for point-of-care diagnostics.
The instruments & analyzers segment is expected to witness the fastest CAGR from 2025 to 2032 due to ongoing innovations aimed at enhancing automation, multiplexing capacity, and user-friendly interfaces, which improve testing efficiency and throughput. In addition, the software & services segment is growing as it provides vital data management, cloud connectivity, and result interpretation solutions that enhance the usability of portable molecular platforms
- By Technology
On the basis of technology, the market is segmented into polymerase chain reaction (PCR), isothermal nucleic acid amplification technology (INAAT), genetic sequencing, and hybridization & microarray technologies. PCR holds the largest market share of 64.7% in 2024, owing to its high sensitivity, specificity, and extensive application in infectious disease detection and genetic testing. PCR’s well-established protocol and reliability make it the preferred choice among healthcare providers.
The INAAT segment is expected to experience the fastest growth from 2025 to 2032, as this technology offers rapid nucleic acid amplification without the need for complex thermal cycling, allowing easier deployment in decentralized, resource-limited, and field settings. Genetic sequencing and hybridization & microarray technologies, while smaller in market share, are growing steadily due to their use in advanced diagnostics and personalized medicine.
- By Application
On the basis of application, the market is segmented into infectious diseases, oncology, hematology, endocrinology, and prenatal testing. Infectious diseases account for the largest share in 2024, driven by the urgent need for rapid and accurate detection of pathogens, especially in light of recent global health challenges. This segment benefits from continuous government and private sector funding to enhance disease surveillance and control.
Oncology is expected to register fastest growth during the forecast period, fueled by increasing awareness of early cancer detection and the rising demand for personalized medicine approaches that rely on molecular diagnostics for targeted therapy decisions. Hematology and endocrinology segments are also expanding as molecular platforms enable improved diagnosis and monitoring of blood disorders and hormonal imbalances. Prenatal testing is gradually gaining traction due to rising demand for non-invasive, early genetic screening
- By End User
On the basis of end user, the market is segmented into hospitals & clinics, diagnostic laboratories, and homecare & assisted living facilities. Hospitals & clinics dominate the market with the largest revenue share in 2024, driven by their critical role in delivering high volumes of diagnostic testing and urgent patient care requiring rapid molecular results. Diagnostic laboratories hold a significant share due to their capacity for high-throughput molecular testing and advanced laboratory infrastructure
The homecare & assisted living facilities segment is expected to witness the fastest CAGR from 2025 to 2032, supported by rising patient preference for decentralized care, aging populations, and advances in user-friendly portable platforms that enable molecular testing outside traditional clinical environments
- By Test Location
On the basis of test location, the market is segmented into over-the-counter (OTC) and point-of-care (PoC). The point-of-care segment dominates the market with the largest share in 2024, supported by the increasing demand for rapid, onsite molecular diagnostic results that facilitate timely clinical decision-making. PoC testing is preferred in hospitals, clinics, and remote locations where access to centralized labs is limited
The OTC segment is expected to witness the fastest CAGR from 2025 to 2032, as consumer awareness and acceptance of molecular self-testing solutions increase, enabling individuals to perform diagnostics conveniently at home for various conditions including infectious diseases. Advances in assay simplification and digital result interpretation are further driving the adoption of OTC molecular tests
Portable Molecular Platforms Market Regional Analysis
- North America dominates the portable molecular platforms market with the largest revenue share of 46.5% in 2024, driven by region's advanced healthcare infrastructure, high adoption rates of innovative diagnostic tools, and the presence of key industry players
- Consumers and healthcare providers in the region highly value the convenience, speed, and precision offered by portable molecular platforms for early disease detection and management
- This widespread adoption is further supported by advanced healthcare infrastructure, strong government initiatives promoting molecular diagnostics, and growing preference for point-of-care testing, establishing portable molecular platforms as the modern diagnostic choice for hospitals, clinics, and laboratories
U.S. Portable Molecular Platforms Market Insight
The U.S. portable molecular platforms market captured the largest revenue share in 2024, fueled by the rapid adoption of advanced molecular diagnostic technologies and growing demand for point-of-care testing. Healthcare providers increasingly prioritize fast and accurate diagnostic solutions to improve patient outcomes. The rising prevalence of infectious diseases, along with government initiatives supporting molecular diagnostics, further propels market growth. Integration with digital health platforms and mobile connectivity enhances usability and adoption across hospitals and diagnostic labs.
Europe Portable Molecular Platforms Market Insight
The Europe portable molecular platforms market is projected to expand at a significant CAGR throughout the forecast period, driven by stringent regulatory frameworks promoting early disease detection and precision medicine. Increasing investments in healthcare infrastructure and rising awareness of molecular diagnostics are boosting demand. The region’s growing focus on decentralizing healthcare and expanding point-of-care testing in both clinical and home settings supports market expansion, with applications spanning infectious diseases and oncology.
U.K. Portable Molecular Platforms Market Insight
The U.K. portable molecular platforms market is anticipated to grow steadily, driven by the emphasis on improving diagnostic turnaround times and enhancing healthcare accessibility. Increased government funding and public-private partnerships are accelerating the adoption of portable diagnostic tools. The rising incidence of chronic and infectious diseases encourages healthcare providers to implement rapid molecular testing solutions, while digital health integration fosters broader acceptance
Germany Portable Molecular Platforms Market Insight
The Germany portable molecular platforms market is expected to grow at a robust CAGR, propelled by a strong healthcare system and commitment to innovation. The country’s emphasis on sustainable and efficient healthcare solutions supports the uptake of portable molecular devices. Growing awareness of personalized medicine and preventive care fuels demand, especially in hospital and diagnostic laboratory settings. Germany’s infrastructure facilitates seamless integration of molecular platforms into existing healthcare workflows
Asia-Pacific Portable Molecular Platforms Market Insight
The Asia-Pacific portable molecular platforms market is poised to grow at the fastest CAGR from 2025 to 2032, driven by rising healthcare expenditures, increasing prevalence of infectious diseases, and expanding healthcare infrastructure in countries such as China, India, and Japan. Government initiatives promoting digital healthcare and rural diagnostics are key growth enablers. The region’s large population base and growing adoption of point-of-care testing contribute significantly to market expansion.
Japan Portable Molecular Platforms Market Insight
The Japan portable molecular platforms market is gaining momentum due to the country’s advanced healthcare technology adoption and aging population demanding rapid diagnostic solutions. Integration with IoT and digital health systems enhances platform utility in clinical and homecare settings. Japan’s focus on precision medicine and infectious disease management further propels market growth, with portable platforms increasingly used in hospitals and assisted living facilities
India Portable Molecular Platforms Market Insight
The India portable molecular platforms market accounted for the largest revenue share in Asia-Pacific in 2024, driven by rising healthcare awareness, rapid urbanization, and increasing access to affordable molecular diagnostics. Government initiatives aimed at improving rural healthcare and the expansion of smart healthcare infrastructure are propelling market growth. The presence of domestic manufacturers and the rising burden of infectious diseases and chronic conditions continue to boost adoption across hospitals, clinics, and point-of-care locations
Portable Molecular Platforms Market Share
The portable molecular platforms industry is primarily led by well-established companies, including:
- Thermo Fisher Scientific Inc. (U.S.)
- QIAGEN (Germany)
- F. Hoffmann-La Roche Ltd (Switzerland)
- BIOMÉRIEUx (France)
- Abbott. (U.S.)
- Danaher Corporration (U.S.)
- Hologic, Inc. (U.S.)
- Illumina, Inc. (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- GenMark Diagnostics, Inc. (U.S.)
- Lucira Health, Inc. (U.S.)
- Molbio Diagnostics Pvt. Ltd. (India)
- Sansure Biotech Inc. (China)
- QuantuMDx Group Ltd. (U.K.)
- Abacus Diagnostica Oy (Finland)
- Mobidiag Ltd (acquired by Hologic) (Finland)
- Co-Diagnostics, Inc. (U.S.)
- Alercell, Inc. (U.S.)
- T2 Biosystems, Inc. (U.S.)
- Enigma Diagnostics Limited (U.K.)
Latest Developments in Global Portable Molecular Platforms Market
- In May 2023, At EuroMedLab Rome 2023, Sansure Biotech presented its iPonatic III, a portable molecular workstation designed for point-of-care testing. The device's compact design and wireless connectivity cater to the increasing demand for decentralized diagnostic solutions, enhancing the accessibility and efficiency of molecular diagnostics
- In April 2023, Molbio Diagnostics introduced Truenat H3N2/H1N1, the first point-of-care real-time PCR test approved by India's CDSCO for the differential diagnosis of H3N2 and H1N1 influenza viruses. Delivering results within an hour, this innovation addresses the need for rapid, on-site testing solutions, supporting market growth in infectious disease diagnostics.
- In April 2023, Alercell launched the LENA Molecular Dx Leukemia Platform, comprising 12 next-generation sequencing-based molecular diagnostic tests for early leukemia detection, treatment guidance, and minimal residual disease monitoring. This platform exemplifies the expanding application of NGS in oncology, contributing to the growth of molecular diagnostics in cancer care
- In February 2023, Thermo Fisher Scientific introduced the QuantStudio Absolute Q AutoRun Digital PCR Suite, an automated, high-throughput solution for precise nucleic acid quantification. Designed for extended hands-free operation, this system enhances the precision and sensitivity of molecular diagnostics, supporting advancements in research and clinical applications
- In January 2023, QIAGEN and Helix announced an exclusive partnership to develop next-generation sequencing-based companion diagnostics for hereditary diseases. Leveraging Helix's FDA-authorized laboratory platform and QIAGEN's global regulatory expertise, this collaboration emphasizes the integral role of NGS in early disease detection and personalized medicine, fostering market expansion
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.