Global Postmenopausal Vaginal Atrophy Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
2.52 Billion
USD
4.50 Billion
2022
2030
USD
2.52 Billion
USD
4.50 Billion
2022
2030
| 2023 –2030 | |
| USD 2.52 Billion | |
| USD 4.50 Billion | |
|
|
|
|
Postmenopausal Vaginal Atrophy Treatment Market Analysis and Size
The growing number of postmenopausal vaginal therapy medications which includes estrogen-based drugs delivered through retail pharmacies is set to enhance the postmenopausal vaginal atrophy treatment market. A number of branded and generic drugs are widely available in the market for the treatment of postmenopausal vaginal atrophy. The development of top advanced treatment methods has also helped the market gain pace in the recent years. In addition to this, the improvement of the healthcare reforms for meeting the requirements for the treatment of vaginal atrophy is also a major factor boosting the development of the market.
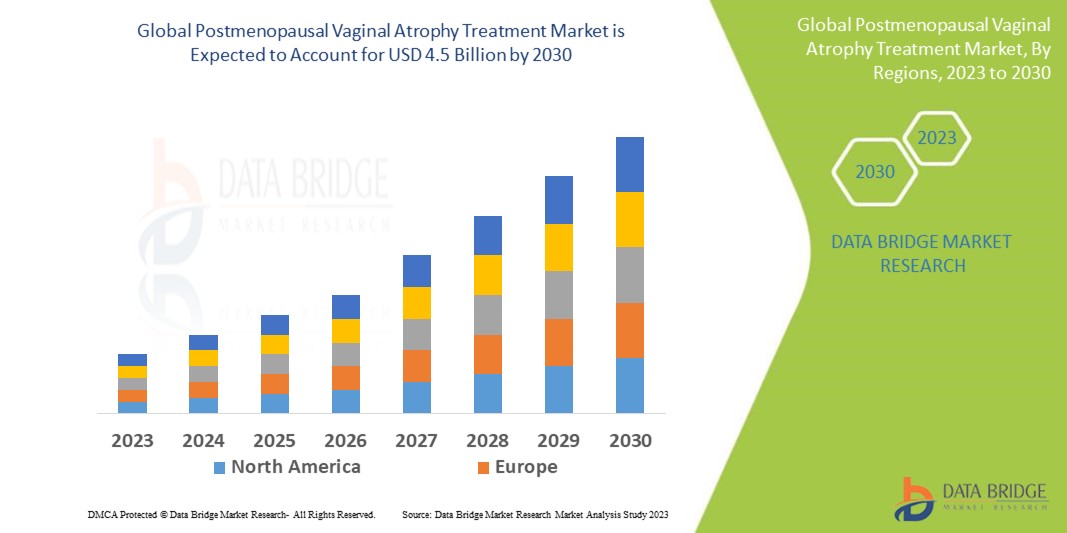
Data Bridge Market Research analyses a growth rate in postmenopausal vaginal atrophy treatment market in the forecast period 2023-2030. The expected CAGR of postmenopausal vaginal atrophy treatment market is tend to be around 7.50% in the mentioned forecast period. The market value is USD 2.52 billion in 2022, and it would grow upto USD 4.5 billion by 2030. In addition to the market insights such as market value, growth rate, market segments, geographical coverage, market players, and market scenario, the market report curated by the Data Bridge Market Research team also includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Postmenopausal Vaginal Atrophy Treatment Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Billion, Volumes in Units, Pricing in USD |
|
Segments Covered |
Therapy Type (Estrogen based drugs, Non-estrogen based drugs and Others), Drug Form (Vaginal gel, Creams, Tablet and Others), Route of Administration (Oral, Intravaginal and Others), End-Users (Hospitals, Homecare, Specialty Clinics, Others), Distribution Channel (Hospital Pharmacy, Online Pharmacy, Retail Pharmacy) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Pfizer Inc (U.S.), Mylan N.V. (U.S.), Novartis AG (Switzerland), Hikma Pharmaceuticals plc (U.K.), Aurobindo Pharma (India), AbbVie Inc. (U.S.) Melinta Therapeutics, Inc (U.S.), Bristol-Myers Squibb Company (U.S.), GSK plc. (U.K.), Bayer AG (Germany), Lupin (India), Perrigo Company plc (U.S.), Bionovo, Inc. (Saudi Arabia), TherapeuticsMD, Inc. (U.S.), Endoceutics, Inc (Canada), Upsher-Smith Laboratories, LLC (U.S.), Ligand Pharmaceuticals Incorporated (U.S.), Pantarhei Bioscience (Netherlands) |
|
Market Opportunities |
|
Market Definition
Postmenopausal vaginal atrophy is the kind of condition of vaginal wall thinning which is caused by a decreased level of estrogen. The varied changes in the genitourinary tract initiating from a hypoestrogenic state includes reduced vaginal lubrication, thinning of the vaginal epithelium and decreased vaginal vascularization. Continuously pH increases and loss of lactobacilli microflora enables opportunistic colonization by pathogenic bacteria; this can produce infection.
Postmenopausal Vaginal Atrophy Treatment Market Dynamics
Drivers
- Incidence of Postmenopausal Vaginal Atrophy
The growing incidence of postmenopausal vaginal atrophy is increasing the demand for treatment methods. The most visible symptom is vaginal dryness which is affecting around 60% of women in the postmenopausal period. In general, these symptoms can cause sexual dysfunction and consequently lead to reduced quality of life. Thus, this factor increases the market growth.
- Increasing Healthcare Plans
Various established healthcare infrastructures, the increased frequency of disease, and the incidence of major manufacturers are the main factors leading to the market share. For instance, The Affordable Care Act has increased the number of insured women and has been a major factor in the treatment-seeking behavior of people suffering from this condition. Additionally, this law offers many U.S. citizens better health security by providing comprehensive health insurance reforms that not only have reduced health care costs but also enhance coverage. Therefore, the launch of beneficial healthcare plans in the U.S. will influence more women to seek treatment, thus helping in the growth of this market during the forecast period 2023-2030.
Opportunities
- Increased Utilization of Vaginal Tablets
Postmenopausal vaginal atrophy may result in symptoms of urogenital atrophy. The launch of the ultra-low-dose 10-µg estradiol vaginal tablets correlates with the needs of regulatory agencies and women's health societies associated with applying the least efficient hormonal dose. The 10-µg estradiol vaginal tablet may offer better reassurance to healthcare providers and postmenopausal women because of its efficiency, safety profiles, and minimal systemic absorption, with an annual estradiol administration of only 1.14 mg. Thus, this factor significantly increases the market growth.
- Increasing Demand of Estrogen Based Drugs
The increasing demand of estrogen-based drugs contributes in leading the market growth. This growth is due to the increasing application of estrogen-based drugs used for the treatment of postmenopausal vaginal atrophy among women. Systemic estrogen treatments includes creams, vaginal gels and patches. The huge application of topical estrogen therapy in the management of postmenopausal vaginal atrophy is boosting the growth of the market. These treatment methods are highly applicable in treating diseases such as hot flashes, urogenital atrophy and others.
Restraints/Challenges
- Lack of Awareness About The Infection
The lack of awareness about these infections could reduce the growth of the global postmenopausal vaginal atrophy treatment market during the forecast period 2023-2030. Not much awareness about the infection leads to improper treatment in several underdeveloped and developing countries thus restraining the market growth.
- Adverse Effects of Vaginal Gels
There are several adverse effects associated with the treatment types of postmenopausal vaginal atrophy. Metronidazole Vaginal Gel is the intravaginal dosage form of the synthetic antibacterial agent, metronidazole, USP at a concentration of 0.75%. Vaginal gels such as Metrogel have few side effects. Metrogel – vaginal gel with applicator can have side effects such as headache, stomach discomfort, or dizziness. Vaginal discomfort or itching and may be discharge may occur or worsen. These symptoms may happen because of new vaginal infection such as yeast or fungal infection. In case of symptoms associated with severe allergic reaction which involves serious dizziness, medical assistance is required immediately. Thus, all these side-effects lead to the drop in usage of vaginal gels or drugs and which in turn decrease the market growth.
This postmenopausal vaginal atrophy treatment market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the postmenopausal vaginal atrophy treatment market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on Global Postmenopausal Vaginal Atrophy Treatment Market
The COVID-19 pandemic has resulted in the hindrance of the development, production, and supply of drugs and other healthcare products and majorly affected the growth of healthcare businesses of many companies globally. Though, in the post-pandemic era, the market is anticipated to grow due to a major increase in R&D initiatives for exact diagnosis. Several government and non-government bodies, including the European Institute of Women's Health (EIWH) and the World Health Organization, have launched many public awareness campaigns about women's health. Therefore, COVID-19 had a major impact on the global postmenopausal vaginal atrophy treatment market.
Recent Developments
- In 2022, Novo Nordisk India launched a first-of-its-kind diabetes treatment medicine in the nation. The firm pronounced that it had launched the world's earliest and only oral semaglutide. It is developed in oral form for the first time.
- In 2021, Pfizer Inc. announced that it acquired Amplyx Pharmaceuticals, Inc. Amplyx’s lead compound, Fosmanogepix (APX001). Fosmanogepix (APX001) is a novel investigational resource under development used for the treatment of invasive fungal infections.
- In 2020, Upsher-Smith Laboratories, LLC announced that it enlarged its generic portfolio with the launch of three novel products. The products were Haloperidol Tablets, USP Fluvoxamine Maleate Tablets, and Clonidine Hydrochloride Extended-release Tablets.
Global Postmenopausal Vaginal Atrophy Treatment Market Scope
The postmenopausal vaginal atrophy treatment market is segmented on the basis of therapy type, drug form, route of administration, distribution channel and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Therapy Type
- Estrogen based drugs
- Non-estrogen based drugs
- Others
Drug Form
- Vaginal gel
- Creams
- Tablet
- Others
Route of Administration
- Oral
- Intravaginal
- Others
End User
- Hospitals
- Homecare
- Specialty Clinics
- Others
Distribution Channel
- Hospital Pharmacy
- Online Pharmacy
- Retail Pharmacy
Postmenopausal Vaginal Atrophy Treatment Market Regional Analysis/Insights
The postmenopausal vaginal atrophy treatment market is analyzed and market size insights and trends are provided by therapy type, drug form, route of administration, distribution channel and end-user as referenced above.
The major countries covered in the postmenopausal vaginal atrophy treatment market report are the U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
Asia-Pacific has been witnessing a positive growth for postmenopausal vaginal atrophy treatment market throughout the forecast period because of growing initiatives taken by the government and pharmaceutical organizations to spread awareness and increased incidence of vaginal infection in women.
North America dominates the market because of growing initiatives taken by the pharmaceutical organizations to produce novel formulation and well-spread awareness of anti-infectives among end-users.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Global Postmenopausal Vaginal Atrophy Treatment Market Share Analysis
The postmenopausal vaginal atrophy treatment market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related postmenopausal vaginal atrophy treatment market.
Key players operating in the postmenopausal vaginal atrophy treatment market include:
- Pfizer Inc (U.S.)
- Mylan N.V. (U.S.)
- Novartis AG (Switzerland)
- Hikma Pharmaceuticals plc (U.K.)
- Aurobindo Pharma (India)
- AbbVie Inc. (U.S.)
- Melinta Therapeutics, Inc (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- GSK plc. (U.K.)
- Bayer AG (Germany)
- Lupin (India)
- Perrigo Company plc (U.S.)
- Bionovo, Inc. (Saudi Arabia)
- TherapeuticsMD, Inc. (U.S.)
- Endoceutics, Inc (Canada)
- Upsher-Smith Laboratories, LLC (U.S.)
- Ligand Pharmaceuticals Incorporated (U.S.)
- Pantarhei Bioscience (Netherlands)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.