Global Pralidoxime Chloride Market
Market Size in USD Billion
CAGR :
% 
 USD
1.38 Billion
USD
1.97 Billion
2025
2033
USD
1.38 Billion
USD
1.97 Billion
2025
2033
| 2026 –2033 | |
| USD 1.38 Billion | |
| USD 1.97 Billion | |
|
|
|
|
Pralidoxime Chloride Market Size
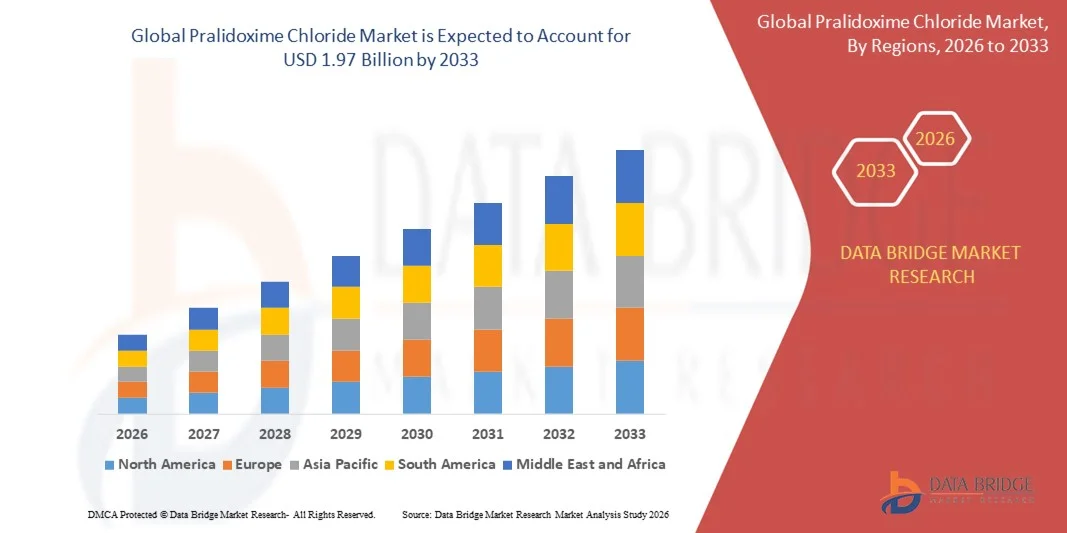
- The global pralidoxime chloride market size was valued at USD 1.38 billion in 2025 and is expected to reach USD 1.97 billion by 2033, at a CAGR of 4.5% during the forecast period
- The market growth is largely fueled by the increasing prevalence of organophosphate pesticide use and the rising incidence of chemical and nerve agent poisoning, driving consistent demand for effective antidotes such as Pralidoxime Chloride
- Furthermore, expanding awareness of poisoning management protocols, emergency preparedness initiatives, and inclusion of Pralidoxime Chloride in hospital essential drug lists are establishing it as a critical treatment option in healthcare and research settings. These factors are accelerating the adoption of Pralidoxime Chloride globally, thereby significantly boosting the market’s growth
Pralidoxime Chloride Market Analysis
- Pralidoxime Chloride, used as an antidote for organophosphate and nerve agent poisoning, is increasingly vital in hospitals, emergency response units, and toxicology research laboratories due to its rapid action, reliability, and integration into standard treatment protocols
- The escalating demand for Pralidoxime Chloride is primarily fueled by the growing awareness of chemical poisoning risks, increased regulatory focus on public safety, and rising investments in healthcare infrastructure and toxicology research across both developed and emerging markets
- North America dominated the pralidoxime chloride market with a share of over 45% in 2025, due to rising awareness of organophosphate poisoning management, increasing prevalence of pesticide exposure incidents, and well-established healthcare infrastructure
- Asia-Pacific is expected to be the fastest growing region in the pralidoxime chloride market during the forecast period due to rising pesticide use in agriculture, increasing awareness of poisoning management, and expanding healthcare and research facilities in countries such as China, Japan, and India
- Injectable segment dominated the market with a market share of 55.5% in 2025, due to its rapid onset of action and suitability for emergency treatment of acute organophosphate poisoning. Healthcare providers prefer injectable formulations due to precise dosing and immediate therapeutic efficacy, which is critical in life-threatening cases. In addition, injectables allow administration in both hospital and pre-hospital settings, reinforcing their widespread adoption
Report Scope and Pralidoxime Chloride Market Segmentation
|
Attributes |
Pralidoxime Chloride Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Pralidoxime Chloride Market Trends
Growing Adoption of Advanced Autoinjector and Ready-To-Use Formulations
- A significant trend in the pralidoxime chloride market is the increasing adoption of advanced autoinjector systems and ready-to-use formulations, driven by the need for rapid and accurate treatment in cases of organophosphate and nerve agent poisoning. These innovations are enhancing emergency response capabilities and improving patient outcomes in both military and civilian settings
- For instance, Meridian Medical Technologies, a Pfizer company, provides prefilled pralidoxime chloride autoinjectors that allow immediate administration in emergency scenarios. Such solutions reduce the risk of dosing errors and ensure rapid delivery of antidotes under critical conditions
- Healthcare providers and first responders are emphasizing portable and easy-to-administer delivery systems, as autoinjectors and prefilled syringes simplify field deployment and reduce the dependency on trained personnel for preparation. This trend is supporting faster intervention in toxic exposure incidents
- The pharmaceutical industry is focusing on ready-to-use liquid formulations that eliminate the need for reconstitution, thereby shortening response times and enhancing patient safety. These formulations are critical in scenarios where every second counts, particularly in mass exposure or industrial accidents
- Military and defense organizations are increasingly integrating these formulations into nerve agent antidote kits, as standardized autoinjectors and prefilled devices streamline logistics, storage, and training. This adoption reinforces the strategic importance of efficient antidote delivery systems
- The market is witnessing growing demand for user-friendly devices that ensure precise dosing, maintain stability under diverse environmental conditions, and support rapid deployment in emergencies. This trend is solidifying advanced autoinjectors and ready-to-use formulations as essential components of modern toxic exposure management
Pralidoxime Chloride Market Dynamics
Driver
Rising Incidence of Organophosphate and Nerve Agent Poisoning
- The growing prevalence of organophosphate poisoning in agricultural regions and the potential threat of nerve agent exposure are driving demand for effective pralidoxime chloride therapies. Rapid administration of antidotes is essential to prevent long-term neurological damage and fatalities
- For instance, the World Health Organization (WHO) reports high rates of organophosphate poisoning in Southeast Asia, emphasizing the urgent need for accessible and reliable pralidoxime chloride solutions. This instance underscores the importance of widespread availability and rapid-response mechanisms
- Increasing use of pesticides in agriculture and the associated risk of accidental or intentional exposure are expanding the market for antidote treatments that can be administered quickly in field conditions. Early intervention through ready-to-use formulations reduces morbidity and improves recovery rates
- Hospitals and emergency medical services are prioritizing stocking autoinjectors and prefilled antidotes to respond to acute poisoning cases efficiently. This focus is enhancing patient survival rates and supporting broader public health preparedness
- Regulatory support and government programs aimed at improving emergency toxicology response further boost market growth. The combination of rising exposure incidents and structured antidote deployment strategies continues to strengthen the driver for pralidoxime chloride adoption
Restraint/Challenge
Limited Manufacturing Capacity and High Production Costs
- The pralidoxime chloride market faces challenges from limited manufacturing capacity and the high costs associated with producing specialized autoinjectors and ready-to-use formulations. Precision engineering, stringent quality standards, and compliance with regulatory requirements elevate overall production complexity
- For instance, companies such as Meridian Medical Technologies invest heavily in cleanroom facilities and validated production lines to meet autoinjector quality standards. These investments increase production expenses and restrict rapid scaling of supply
- The need for stable formulations that retain efficacy under varying temperature and storage conditions adds further complexity to manufacturing. Maintaining consistent potency across batches requires rigorous testing and monitoring, which drives operational costs
- Limited availability of raw materials and specialized components used in autoinjector devices constrains production flexibility and can lead to supply shortages during sudden demand spikes. This challenge emphasizes the need for careful supply chain management
- Meeting regulatory approvals for emergency-use pharmaceuticals involves extensive clinical testing, documentation, and compliance measures. These requirements extend lead times and increase financial burden for manufacturers, collectively restraining market expansion
Pralidoxime Chloride Market Scope
The market is segmented on the basis of application, formulation type, end use industry, and distribution channel.
- By Application
On the basis of application, the Pralidoxime Chloride market is segmented into pharmaceuticals, agriculture, chemical research, and toxicological analysis. The pharmaceuticals segment dominated the market with the largest revenue share in 2025, driven by its widespread use in treating organophosphate poisoning and its critical role in emergency medical care. Hospitals and clinics often prioritize Pralidoxime Chloride for its rapid action in reversing neuromuscular effects caused by poisoning, making it a standard treatment in toxicology wards. Its inclusion in essential drug lists in several countries further reinforces the segment’s strong demand and consistent market growth.
The toxicological analysis segment is anticipated to witness the fastest growth rate from 2026 to 2033, fueled by increasing research on pesticide exposure, chemical safety, and poisoning diagnostics. Laboratories are adopting advanced analytical techniques to monitor organophosphate residues, and Pralidoxime Chloride is frequently used in controlled experimental studies. The segment’s growth is further supported by heightened regulatory focus on public safety and the expansion of toxicology research initiatives globally.
- By Formulation Type
On the basis of formulation type, the market is segmented into injectable, tablet, and oral solution forms. The injectable segment dominated the market with the largest share of 55.5% in 2025, owing to its rapid onset of action and suitability for emergency treatment of acute organophosphate poisoning. Healthcare providers prefer injectable formulations due to precise dosing and immediate therapeutic efficacy, which is critical in life-threatening cases. In addition, injectables allow administration in both hospital and pre-hospital settings, reinforcing their widespread adoption.
The oral solution segment is expected to witness the fastest growth rate from 2026 to 2033, driven by increasing demand for patient-friendly and non-invasive administration methods. Oral solutions are particularly preferred in outpatient settings, pediatric care, and for long-term management of low-level exposure cases. Pharmaceutical companies are investing in the development of stable and palatable oral solutions, enhancing patient compliance and expanding market opportunities.
- By End Use Industry
On the basis of end use industry, the market is segmented into healthcare, research laboratories, and the agricultural sector. The healthcare industry dominated the market in 2025, driven by the critical role of Pralidoxime Chloride in treating pesticide and nerve agent poisoning. Hospitals, emergency response units, and clinics rely on consistent availability to manage acute toxicity cases effectively. Furthermore, integration into national antidote stockpiles and hospital pharmacy protocols ensures steady consumption and market stability.
The research laboratories segment is anticipated to witness the fastest growth from 2026 to 2033, fueled by rising investments in toxicology research and organophosphate-related studies. Laboratories require Pralidoxime Chloride for experimental work, in vitro testing, and pharmacological research. Increasing focus on chemical safety, pesticide exposure studies, and drug development is expanding demand within this sector.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into online sales, pharmacies, and direct sales. Pharmacies dominated the market in 2025, driven by the widespread accessibility and reliability of traditional pharmaceutical distribution networks. Hospitals and emergency care facilities often source Pralidoxime Chloride through established pharmacy channels for assured quality and regulatory compliance. Pharmacies also play a pivotal role in maintaining inventory for both acute treatment needs and outpatient prescriptions.
The online sales segment is expected to witness the fastest growth rate from 2026 to 2033, driven by the increasing adoption of digital procurement platforms in healthcare and research industries. Online channels offer convenience, faster delivery, and bulk ordering options for hospitals, clinics, and laboratories. The growth of e-pharmacies and specialized chemical suppliers is further facilitating broader market reach and accessibility.
Pralidoxime Chloride Market Regional Analysis
- North America dominated the pralidoxime chloride market with the largest revenue share of over 45% in 2025, driven by rising awareness of organophosphate poisoning management, increasing prevalence of pesticide exposure incidents, and well-established healthcare infrastructure
- Healthcare providers in the region prioritize rapid-access antidotes, emergency care protocols, and the inclusion of Pralidoxime Chloride in hospital essential drug lists, ensuring consistent demand
- The widespread adoption is further supported by advanced research facilities, high healthcare spending, and growing government initiatives for toxicological preparedness, establishing North America as a leading market for both hospital and research applications
U.S. Pralidoxime Chloride Market Insight
The U.S. market captured the largest revenue share in 2025 within North America, fueled by the increasing prevalence of chemical poisoning cases and the prioritization of antidote availability in hospitals and emergency care centers. Rapid response to organophosphate poisoning, combined with strong regulatory frameworks and awareness campaigns, drives adoption. Research institutions also contribute to demand through pharmacological and toxicological studies. The growth of online pharmaceutical distribution channels and established pharmacy networks further enhances accessibility and market penetration.
Europe Pralidoxime Chloride Market Insight
The Europe market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by stringent chemical safety regulations and increased focus on occupational and environmental exposure monitoring. The region sees steady adoption in healthcare, research laboratories, and toxicological studies. Urbanization, growing agricultural practices, and investments in healthcare infrastructure support the steady demand for Pralidoxime Chloride. Countries such as Germany, France, and Italy are actively incorporating antidotes into hospital emergency protocols, further reinforcing market growth.
U.K. Pralidoxime Chloride Market Insight
The U.K. market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by rising awareness of organophosphate exposure risks and emergency treatment preparedness. Hospitals and clinics are increasingly stocking Pralidoxime Chloride to manage poisoning incidents, and research organizations are investing in toxicological studies. The adoption of advanced healthcare technologies and a well-established pharmaceutical distribution network are supporting market expansion.
Germany Pralidoxime Chloride Market Insight
The Germany market is expected to expand at a considerable CAGR during the forecast period, fueled by high awareness of chemical safety, robust healthcare infrastructure, and growing research initiatives in toxicology. The country’s strong emphasis on emergency preparedness, occupational safety, and laboratory research promotes adoption of Pralidoxime Chloride. Hospitals and research labs are increasingly integrating it into treatment protocols and controlled studies, supporting consistent market demand.
Asia-Pacific Pralidoxime Chloride Market Insight
The Asia-Pacific market is poised to grow at the fastest CAGR during the forecast period of 2026 to 2033, driven by rising pesticide use in agriculture, increasing awareness of poisoning management, and expanding healthcare and research facilities in countries such as China, Japan, and India. Government initiatives to improve rural healthcare access and emergency response systems are supporting adoption. Growing pharmaceutical manufacturing capabilities in the region also enhance availability and affordability of Pralidoxime Chloride, expanding its reach across healthcare and research sectors.
Japan Pralidoxime Chloride Market Insight
The Japan market is gaining momentum due to stringent chemical safety regulations, advanced healthcare systems, and a growing focus on toxicological research. Hospitals prioritize ready availability of antidotes for emergency care, while research labs are using Pralidoxime Chloride for controlled studies. The country’s technological advancements and focus on efficient healthcare delivery are driving demand in both residential and institutional applications.
China Pralidoxime Chloride Market Insight
The China market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to widespread pesticide use, rapid urbanization, and a growing healthcare infrastructure. Hospitals and clinics are increasingly stocking Pralidoxime Chloride for emergency response, and research laboratories are adopting it for toxicological studies. Government programs promoting chemical safety and the presence of domestic manufacturers producing affordable solutions are key factors propelling the market in China.
Pralidoxime Chloride Market Share
The pralidoxime chloride industry is primarily led by well-established companies, including:
- Baxter International Inc. (U.S.)
- Meridian Medical Technologies, Inc. (U.S.)
- Taj Pharmaceuticals Ltd. (India)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Pfizer Inc. (U.S.)
- Merck KGaA (Germany)
- Sun Pharmaceutical Industries Ltd. (India)
- Beijing Wellso Pharmaceutical (China)
- China Res Double‑Crane Inc. (China)
- Hikma Pharmaceuticals PLC (U.K.)
- Amphastar Pharmaceuticals, Inc. (U.S.)
- Mylan N.V. (Viatris) (Netherlands/U.S.)
- Incepta Pharmaceuticals Ltd. (Bangladesh)
- Livealth Biopharma (India)
- Paxter Lifesciences (India)
Latest Developments in Global Pralidoxime Chloride Market
- In January 2026, the United Kingdom signed a contract for the supply of DUODOTE® (atropine and pralidoxime chloride auto-injector) to maintain national emergency antidote stockpiles. This development strengthens the market by increasing demand for ready-to-use autoinjector solutions, highlighting the importance of integrated delivery systems in emergency healthcare. The move reflects governments’ focus on rapid response to chemical exposure incidents, encouraging manufacturers to invest in production capacity and improve product availability for hospitals and emergency care units
- In November 2025, the United Kingdom Health Security Agency announced plans to procure combined autoinjectors containing pralidoxime chloride and atropine. This initiative supports market growth by emphasizing advanced, easy-to-administer antidote formats that enhance treatment efficiency in critical scenarios. It encourages adoption of combined formulations in both public health preparedness and hospital emergency kits, driving innovation in delivery technologies and expanding the market scope beyond traditional injectable forms
- In July 2025, Teva Pharmaceuticals entered a strategic partnership with a regional injectables manufacturer to increase supply and distribution of pralidoxime chloride injections. This partnership strengthens the market by ensuring reliable availability of this critical antidote in previously underserved regions. It addresses supply chain challenges, supports growing demand from hospitals and research laboratories, and facilitates broader penetration of injectable formulations, thereby enhancing overall market stability and reliability
- In March 2025, Mylan (under Viatris) completed the acquisition of a specialized injectables manufacturer, including its pralidoxime chloride production capabilities. This acquisition expands the company’s product portfolio, improving its competitive position in the emergency antidote segment. The development also contributes to market growth by consolidating manufacturing expertise, ensuring consistent quality, and enabling higher production capacity to meet rising global demand for rapid-response antidotes in healthcare and research applications
- In February 2025, Pfizer launched a new generic pralidoxime chloride injectable in select markets after regulatory approval. This launch improves accessibility of the antidote, making treatment of organophosphate poisoning more widely available and affordable. The development stimulates market growth by increasing competition, encouraging adoption in hospitals and emergency care facilities, and supporting expansion into emerging regions where access to critical antidotes is limited
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Global Pralidoxime Chloride Market, Supply Chain Analysis and Ecosystem Framework
To support market growth and help clients navigate the impact of geopolitical shifts, DBMR has integrated in-depth supply chain analysis into its Global Pralidoxime Chloride Market research reports. This addition empowers clients to respond effectively to global changes affecting their industries. The supply chain analysis section includes detailed insights such as Global Pralidoxime Chloride Market consumption and production by country, price trend analysis, the impact of tariffs and geopolitical developments, and import and export trends by country and HSN code. It also highlights major suppliers with data on production capacity and company profiles, as well as key importers and exporters. In addition to research, DBMR offers specialized supply chain consulting services backed by over a decade of experience, providing solutions like supplier discovery, supplier risk assessment, price trend analysis, impact evaluation of inflation and trade route changes, and comprehensive market trend analysis.
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.