Global Prion Disease Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
5.14 Billion
USD
7.53 Billion
2024
2032
USD
5.14 Billion
USD
7.53 Billion
2024
2032
| 2025 –2032 | |
| USD 5.14 Billion | |
| USD 7.53 Billion | |
|
|
|
|
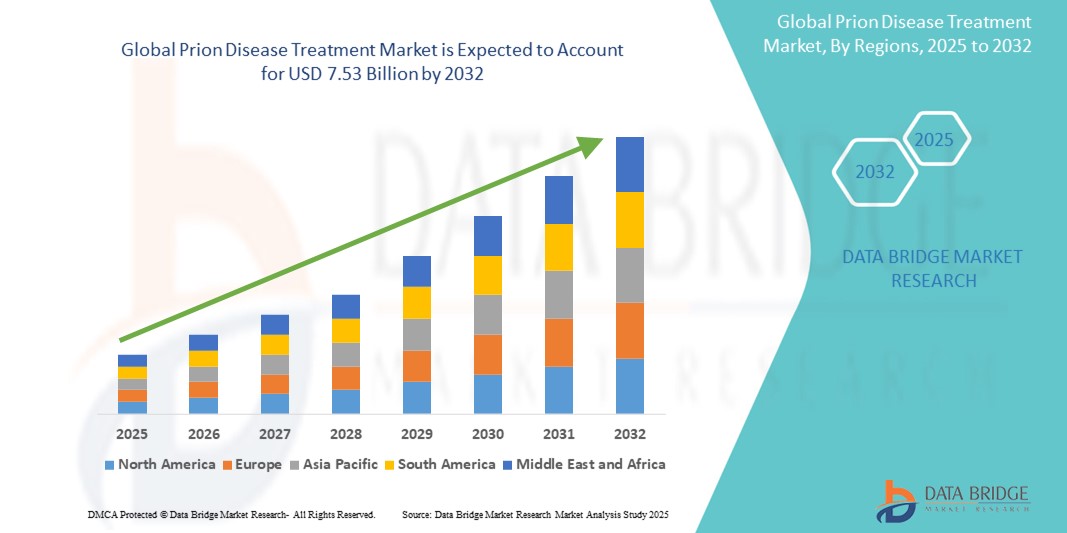
Prion Disease Treatment Market Size
- The global prion disease Treatment market was valued at USD 5.14 billion in 2024 and is expected to reach USD 7.53 billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 4.90%, primarily driven by the increasing prevalence of prion diseases
- This growth is driven by factors such as increased funding for rare diseases, and advancements in technology
Prion Disease Treatment Market Analysis
- Prion diseases are a group of rare, fatal neurodegenerative disorders caused by misfolded proteins, including Creutzfeldt-Jakob disease (CJD), Bovine Spongiform Encephalopathy (BSE), and Fatal Familial Insomnia (FFI). The treatment market for prion diseases focuses on managing symptoms and slowing disease progression, as no definitive cure exists
- The demand for treatments is primarily driven by the increasing diagnosis and awareness of prion diseases. Although rare, these diseases have high fatality rates and limited therapeutic options, resulting in significant market potential for the development of novel treatments. Research in prion diseases is advancing, with new therapies being explored, such as protein-targeting drugs, gene therapy, and immunotherapies
- North America is a dominant region in the prion disease treatment market, owing to its robust healthcare infrastructure, increased research funding, and government support for rare disease treatments. U.S.-based institutions and pharmaceutical companies are leading research into prion disease therapies, driving market growth
- For instance, the U.S. National Institutes of Health (NIH) has allocated research funding specifically for prion diseases, fostering advancements in diagnostics and therapeutics
- Globally, the prion disease treatment market remains underdeveloped compared to other disease areas due to the rarity of prion diseases and the complex nature of prion proteins. However, emerging research and innovations in biotechnology are creating significant opportunities for market growth. With ongoing advancements in research and increasing funding for rare disease therapies, the prion disease treatment market is expected to expand significantly in the coming years
Report Scope and Prion Disease Treatment Market Segmentation
|
Attributes |
Prion Disease Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Prion Disease Treatment Market Trends
“Increasing Adoption of Advanced Diagnostics and Therapeutics in Prion Disease Treatment”
- One prominent trend in the global prion disease treatment market is the growing adoption of advanced diagnostics and therapeutics aimed at better understanding and targeting prion diseases
- With advancements in molecular diagnostics, researchers are now able to detect prion diseases in earlier stages, allowing for more effective intervention and personalized treatment plans
- For instance, innovations such as real-time PCR tests and biomarkers specific to prion diseases are enhancing diagnostic precision and enabling the development of treatments that can specifically target prion proteins or modify their misfolding processes
- In addition, targeted therapeutic strategies, such as gene therapies or prion protein inhibitors, are gaining traction in clinical trials, offering hope for more effective treatments
- This trend is improving the outcomes for patients with prion diseases and driving the demand for new, technologically advanced treatments in the market
Prion Disease Treatment Market Dynamics
Driver
“Growing Awareness and Research Advancements in Prion Disease Treatment”
- The increasing awareness of prion diseases, such as Creutzfeldt-Jakob disease (CJD), and the rising research efforts aimed at understanding the pathogenesis of these diseases are major factors driving the prion disease treatment market
- The growing understanding of prion diseases has led to better diagnostic techniques, increased funding for research, and the development of novel therapies, which is expanding treatment options for affected individuals
- As scientists explore various approaches, including gene therapy, prion inhibitors, and immune-modulatory treatments, the demand for effective treatments continues to grow, prompting the development of new treatment protocols
- The rising focus on personalized medicine and tailored approaches for prion diseases contributes to the overall market growth, improving the effectiveness of treatments for patients
For instance:
- In August 2024, the World Health Organization published a report focusing on research initiatives that aim to understand and treat prion diseases. Increased investment in these research efforts is directly contributing to the growth of the prion disease treatment market
- The prion disease treatment market is driven by increased awareness, research advancements, and the development of novel therapies, including gene therapy and personalized medicine
Opportunity
“Advancement in Targeted Therapies and Early Diagnostics”
- One significant opportunity in the prion disease treatment market is the advancement in targeted therapies and early diagnostic methods
- The ability to diagnose prion diseases earlier will allow for more effective intervention and potentially slow or halt the progression of these neurodegenerative diseases. This is especially important given the long incubation periods of prion diseases
- Advances in molecular diagnostics, such as real-time PCR and other biomarker-based tests, are facilitating early detection, which can significantly improve treatment outcomes
- In addition, promising therapeutic approaches, including prion protein misfolding inhibitors and gene therapy, offer hope for more effective treatments, especially in clinical trials
For instance:
- In September 2024, a groundbreaking study published by Nature Communications highlighted a potential prion-targeted gene therapy that could significantly slow the progression of prion diseases. Such developments represent an exciting opportunity for the market, as they pave the way for more effective treatment options
- The prion disease treatment market presents an opportunity through advancements in targeted therapies and early diagnostic methods, including gene therapy and biomarker-based tests, improving treatment outcomes
Restraint/Challenge
“Lack of Effective Treatment Options and High R&D Costs”
- One major challenge facing the prion disease treatment market is the lack of effective, approved treatment options for prion diseases. Despite significant research efforts, there are currently no treatments that can effectively cure prion diseases
- The complexity of prion diseases, along with their long incubation periods and unique pathology, makes it difficult to develop viable treatments. This uncertainty hinders market growth
- Furthermore, the high costs of research and development (R&D), coupled with the relatively small patient population, make it challenging for pharmaceutical companies to invest in the necessary resources to develop new therapies
For instance:
- In May 2024, an article published by the Journal of Neurodegenerative Diseases highlighted the substantial cost and time barriers to developing new prion disease treatments, which has slowed the progress of potential therapeutic options
- The prion disease treatment market faces challenges due to the lack of effective treatments, high R&D costs, and the complexity of prion diseases, hindering development progress
Prion Disease Treatment Market Scope
The market is segmented on the basis of types, drug, route of administration and end-user.
|
Segmentation |
Sub-Segmentation |
|
By Types |
|
|
By Drug |
|
|
By Route of Administration |
|
|
By End User |
|
Prion Disease Treatment Market Regional Analysis
“North America is the Dominant Region in the Prion Disease Treatment Market”
- North America leads the prion disease treatment market, driven by robust healthcare infrastructure, high levels of investment in research and development, and the presence of leading pharmaceutical companies focused on prion diseases
- U.S. holds the largest share due to a high prevalence of prion diseases, significant research funding, and continuous advancements in treatment strategies, including gene therapy and prion inhibitors
- The strong regulatory frameworks and reimbursement policies in the region further boost market growth by supporting the development and adoption of new treatments
- Ongoing collaborations between healthcare providers, government organizations, and academic institutions are enhancing the effectiveness of diagnostic techniques and therapeutic approaches for prion diseases.
“Asia-Pacific is Projected to Witness the Highest Growth Rate”
- Asia-Pacific is expected to experience the highest growth rate in the prion disease treatment market, driven by the growing healthcare infrastructure, increasing research on neurodegenerative diseases, and rising awareness of prion diseases
- Countries such as China, India, and Japan are emerging as key markets for prion disease treatment due to their large populations, improving healthcare systems, and rising government and private investments in medical research
- Japan, known for its advanced research in neurodegenerative diseases and prion-related studies, remains a crucial player in the development and adoption of prion disease therapies
- China and India are focusing on enhancing their healthcare infrastructure, including research facilities dedicated to rare and neglected diseases, which is expected to significantly contribute to market growth in the coming years
Prion Disease Treatment Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Thermo Fisher Scientific Inc. (U.S.)
- Illumina, Inc. (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Oncocyte Corporation (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- PathAI, Inc. (U.S.)
- Guardant Health (U.S.)
- Labcorp.(U.S.)
- Sysmex Inostics Inc. (U.S.)
- Sangamo Therapeutics, Inc. (U.S.)
- Voyager Therapeutics, Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- Genentech, Inc. (U.S.)
Latest Developments in Global Prion Disease Treatment Market
- In May 2025, researchers at the Broad Institute of MIT and Harvard utilized base editing to introduce a single-letter DNA change, reducing disease-causing prion protein levels in a mouse model by up to 60%, leading to a lifespan extension of approximately 50%
- In April 2025, preclinical studies demonstrated that Antisense Oligonucleotide (ASO) therapy could effectively lower prion protein levels, tripling survival rates in prion-infected animals. A single dose administered after symptom onset prolonged survival by months in some cases
- In March 2025, the development of the CHARMs technology, which silences the gene encoding prion protein, was announced, achieving up to an 80% reduction in prion protein levels in mouse brains
- In February 2025, researchers identified ten compounds capable of reducing prion protein accumulation in infected cells, five of which are already approved for human use, providing an expedited path to clinical application
- In January 2025, Ionis Pharmaceuticals completed enrollment for a Phase 1/2a clinical trial (PrProfile) evaluating ION717 in symptomatic prion disease patients, marking a significant step toward developing effective treatments for prion diseases
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL PRION DISEASE TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL PRION DISEASE TREATMENT MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY BASED MODEL
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL PRION DISEASE TREATMENT MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 PESTEL ANALYSIS
5.2 PORTER’S 5 FORCES
6 EPIDEMIOLOGY
6.1 INCIDENCES & PREVELENCE OF CREUTZFELDT-JAKOB DISEASE [CJD] , BY COUNTRY
6.2 INCIDENCES & PREVELENCE OF SPORADIC FATAL INSOMNIA, BY COUNTRY
6.3 INCIDENCES & PREVELENCE OF VARIABLY PROTEASE-SENSITIVE PRIONOPATHY, BY COUNTRY
6.4 INCIDENCES & PREVELENCE OF GENETIC CJD, BY COUNTRY
6.5 INCIDENCES & PREVELENCE OF FATAL FAMILIAL INSOMNIA, BY COUNTRY
6.6 INCIDENCES & PREVELENCE OF GERSTMANN-STRAUSSLER-SCHEINKER SYNDROME, BY COUNTRY
6.7 INCIDENCES & PREVELENCE OF KURU, BY COUNTRY
6.8 INCIDENCES & PREVELENCE OF VARIANT CJD, BY COUNTRY
6.9 INCIDENCES & PREVELENCE OF IATROGENIC CJD, BY COUNTRY
7 INDUSTRY INSIGHTS
8 REGULATORY FRAMEWORK
9 PIPELINE ANALYSIS
9.1 OVERVIEW
9.2 PHASE III CANDIDATES
9.3 PHASE II CANDIDATES
9.4 PHASE I CANDIDATES
9.5 OTHERS
10 GLOBAL PRION DISEASE TREATMENT MARKET, BY TREATMENT
10.1 OVERVIEW
10.2 THE CENTRAL NERVOUS SYSTEM DRUGS
10.2.1 ANTIEPILEPTIC DRUGS
10.2.1.1. CHLORPROMAZINE
10.2.1.2. TOPIRAMATE
10.2.1.3. PHENYTOIN
10.2.1.4. LEVETIRACETAM
10.2.1.5. OTHERS
10.2.2 ANTIDEPRESSANTS AGENTS
10.2.2.1. CLOMIPRAMINE
10.2.2.2. DESIPRAMINE
10.2.2.3. VENLAFAXINE
10.2.2.4. TRIMIPRAMINE
10.2.2.5. OTHERS
10.2.3 OTHERS
10.3 ANTIVIRALS
10.3.1 ACYCLOVIR
10.3.2 AMANTADINE
10.3.3 INTERFERON
10.3.4 VIDARABINE
10.3.5 OTHERS
10.4 ANTIBIOTIC
10.4.1 DOXYCYCLINE
10.4.2 AMPHOTERICIN B
10.4.3 OTHERS
10.5 FLUPIRTINE
10.6 FLUPHENAZINE
10.7 QUINACRINE
10.8 PENTOSAN POLYSULFATE
10.9 OTHERS
11 GLOBAL PRION DISEASE TREATMENT MARKET, BY TYPE
11.1 OVERVIEW
11.2 SPORADIC
11.2.1 CREUTZFELDT-JAKOB DISEASE [CJD]
11.2.2 SPORADIC FATAL INSOMNIA
11.2.3 VARIABLY PROTEASE-SENSITIVE PRIONOPATHY
11.3 GENETIC
11.3.1 GENETIC CJD
11.3.2 FATAL FAMILIAL INSOMNIA
11.3.3 GERSTMANN-STRAUSSLER-SCHEINKER SYNDROME
11.4 ACQUIRED
11.4.1 KURU
11.4.2 VARIANT CJD
11.4.3 IATROGENIC CJD
12 GLOBAL PRION DISEASE TREATMENT MARKET, BY DRUG TYPE
12.1 OVERVIEW
12.2 BRANDED
12.2.1 ELMIRON
12.2.2 ANAFRANIL
12.2.3 NORPRAMIN
12.2.4 EFFEXOR
12.2.5 EPRONTIA
12.2.6 QSYMIA
12.2.7 ABELCET
12.2.8 AMBISOME
12.2.9 OTHER
12.3 GENERICS
13 GLOBAL PRION DISEASE TREATMENT MARKET, BY AGE GROUP
13.1 OVERVIEW
13.2 PADEATRICS
13.3 ADULTS
13.4 GERIATRICS
14 GLOBAL PRION DISEASE TREATMENT MARKET, BY ROUTE OF ADMINISTRATION
14.1 OVERVIEW
14.2 ORAL
14.2.1 TABLETS
14.2.2 CAPSULE
14.3 INJECTABLES
14.3.1 INTRAMASCULAR
14.3.2 INTRAVENOUS
14.4 OTHERS
15 GLOBAL PRION DISEASE TREATMENT MARKET, BY END USER
15.1 OVERVIEW
15.2 HOSPITALS
15.3 HOMECARE
15.4 SPECIALTY CLINICS
15.5 OTHERS.
16 GLOBAL PRION DISEASE TREATMENT MARKET, BY DISTRIBUTION CHANNEL
16.1 OVERVIEW
16.2 DIRECT TENDER
16.3 RETAIL SALES
16.3.1 ONLINE RETAIL CHANNELS
16.3.2 PHARMACY STORES
16.3.3 OTHERS
16.4 OTHERS
17 GLOBAL PRION DISEASE TREATMENT MARKET, BY GEOGRAPHY
GLOBAL PRION DISEASE TREATMENT MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
17.1.1 NORTH AMERICA
17.1.1.1. U.S.
17.1.1.2. CANADA
17.1.1.3. MEXICO
17.1.2 EUROPE
17.1.2.1. GERMANY
17.1.2.2. U.K.
17.1.2.3. ITALY
17.1.2.4. FRANCE
17.1.2.5. SPAIN
17.1.2.6. RUSSIA
17.1.2.7. SWITZERLAND
17.1.2.8. TURKEY
17.1.2.9. BELGIUM
17.1.2.10. NETHERLANDS
17.1.2.11. DENMARK
17.1.2.12. SWEDEN
17.1.2.13. POLAND
17.1.2.14. NORWAY
17.1.2.15. FINLAND
17.1.2.16. REST OF EUROPE
17.1.3 ASIA-PACIFIC
17.1.3.1. JAPAN
17.1.3.2. CHINA
17.1.3.3. SOUTH KOREA
17.1.3.4. INDIA
17.1.3.5. SINGAPORE
17.1.3.6. THAILAND
17.1.3.7. INDONESIA
17.1.3.8. MALAYSIA
17.1.3.9. PHILIPPINES
17.1.3.10. AUSTRALIA
17.1.3.11. NEW ZEALAND
17.1.3.12. VIETNAM
17.1.3.13. TAIWAN
17.1.3.14. REST OF ASIA-PACIFIC
17.1.4 SOUTH AMERICA
17.1.4.1. BRAZIL
17.1.4.2. ARGENTINA
17.1.4.3. REST OF SOUTH AMERICA
17.1.5 MIDDLE EAST AND AFRICA
17.1.5.1. SOUTH AFRICA
17.1.5.2. EGYPT
17.1.5.3. BAHRAIN
17.1.5.4. UNITED ARAB EMIRATES
17.1.5.5. KUWAIT
17.1.5.6. OMAN
17.1.5.7. QATAR
17.1.5.8. SAUDI ARABIA
17.1.5.9. REST OF MEA SOUTH AFRICA
18 GLOBAL PRION DISEASE TREATMENT MARKET, COMPANY LANDSCAPE
18.1 COMPANY SHARE ANALYSIS: GLOBAL
18.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
18.3 COMPANY SHARE ANALYSIS: EUROPE
18.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
18.5 MERGERS & ACQUISITIONS
18.6 NEW PRODUCT DEVELOPMENT & APPROVALS
18.7 EXPANSIONS
18.8 REGULATORY CHANGES
18.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
19 GLOBAL PRION DISEASE TREATMENT MARKET, SWOT AND DBMR ANALYSIS
20 GLOBAL PRION DISEASE TREATMENT MARKET, COMPANY PROFILE
20.1 TEVA PHARMACEUTICAL INDUSTRIES LTD
20.1.1 COMPANY OVERVIEW
20.1.2 REVENUE ANALYSIS
20.1.3 GEOGRAPHIC PRESENCE
20.1.4 PRODUCT PORTFOLIO
20.1.5 RECENT DEVELOPMENTS
20.2 DR. REDDY’S LABORATORIES LTD.
20.2.1 COMPANY OVERVIEW
20.2.2 REVENUE ANALYSIS
20.2.3 GEOGRAPHIC PRESENCE
20.2.4 PRODUCT PORTFOLIO
20.2.5 RECENT DEVELOPMENTS
20.3 PFIZER INC
20.3.1 COMPANY OVERVIEW
20.3.2 REVENUE ANALYSIS
20.3.3 GEOGRAPHIC PRESENCE
20.3.4 PRODUCT PORTFOLIO
20.3.5 RECENT DEVELOPMENTS
20.4 SANDOZ INTERNATIONAL GMBH
20.4.1 COMPANY OVERVIEW
20.4.2 REVENUE ANALYSIS
20.4.3 GEOGRAPHIC PRESENCE
20.4.4 PRODUCT PORTFOLIO
20.4.5 RECENT DEVELOPMENTS
20.5 SUN PHARMACEUTICAL INDUSTRIES LTD.
20.5.1 COMPANY OVERVIEW
20.5.2 REVENUE ANALYSIS
20.5.3 GEOGRAPHIC PRESENCE
20.5.4 PRODUCT PORTFOLIO
20.5.5 RECENT DEVELOPMENTS
20.6 APOTEX INC.
20.6.1 COMPANY OVERVIEW
20.6.2 REVENUE ANALYSIS
20.6.3 GEOGRAPHIC PRESENCE
20.6.4 PRODUCT PORTFOLIO
20.6.5 RECENT DEVELOPMENTS
20.7 ZYDUS PHARMACEUTICALS INC
20.7.1 COMPANY OVERVIEW
20.7.2 REVENUE ANALYSIS
20.7.3 GEOGRAPHIC PRESENCE
20.7.4 PRODUCT PORTFOLIO
20.7.5 RECENT DEVELOPMENTS
20.8 GLENMARK PHARMACEUTICALS
20.8.1 COMPANY OVERVIEW
20.8.2 REVENUE ANALYSIS
20.8.3 GEOGRAPHIC PRESENCE
20.8.4 PRODUCT PORTFOLIO
20.8.5 RECENT DEVELOPMENTS
20.9 LANNETT
20.9.1 COMPANY OVERVIEW
20.9.2 REVENUE ANALYSIS
20.9.3 GEOGRAPHIC PRESENCE
20.9.4 PRODUCT PORTFOLIO
20.9.5 RECENT DEVELOPMENTS
20.1 CIPLA INC
20.10.1 COMPANY OVERVIEW
20.10.2 REVENUE ANALYSIS
20.10.3 GEOGRAPHIC PRESENCE
20.10.4 PRODUCT PORTFOLIO
20.10.5 RECENT DEVELOPMENTS
20.11 LUPIN
20.11.1 COMPANY OVERVIEW
20.11.2 REVENUE ANALYSIS
20.11.3 GEOGRAPHIC PRESENCE
20.11.4 PRODUCT PORTFOLIO
20.11.5 RECENT DEVELOPMENTS
20.12 ALEMBIC PHARMECAUTICALS
20.12.1 COMPANY OVERVIEW
20.12.2 REVENUE ANALYSIS
20.12.3 GEOGRAPHIC PRESENCE
20.12.4 PRODUCT PORTFOLIO
20.12.5 RECENT DEVELOPMENTS
20.13 APOTEX INC
20.13.1 COMPANY OVERVIEW
20.13.2 REVENUE ANALYSIS
20.13.3 GEOGRAPHIC PRESENCE
20.13.4 PRODUCT PORTFOLIO
20.13.5 RECENT DEVELOPMENTS
20.14 ACCORD HEALTHCARE INC
20.14.1 COMPANY OVERVIEW
20.14.2 REVENUE ANALYSIS
20.14.3 GEOGRAPHIC PRESENCE
20.14.4 PRODUCT PORTFOLIO
20.14.5 RECENT DEVELOPMENTS
20.15 AURBINDO PHARMA LIMITED
20.15.1 COMPANY OVERVIEW
20.15.2 REVENUE ANALYSIS
20.15.3 GEOGRAPHIC PRESENCE
20.15.4 PRODUCT PORTFOLIO
20.15.5 RECENT DEVELOPMENTS
20.16 LEADIANT BIOSCIENCES, INC.
20.16.1 COMPANY OVERVIEW
20.16.2 REVENUE ANALYSIS
20.16.3 GEOGRAPHIC PRESENCE
20.16.4 PRODUCT PORTFOLIO
20.16.5 RECENT DEVELOPMENTS
20.17 ASTELLAS PHARMA US, INC
20.17.1 COMPANY OVERVIEW
20.17.2 REVENUE ANALYSIS
20.17.3 GEOGRAPHIC PRESENCE
20.17.4 PRODUCT PORTFOLIO
20.17.5 RECENT DEVELOPMENTS
20.18 JANSSEN PHARMACEUTICALS, INC.
20.18.1 COMPANY OVERVIEW
20.18.2 REVENUE ANALYSIS
20.18.3 GEOGRAPHIC PRESENCE
20.18.4 PRODUCT PORTFOLIO
20.18.5 RECENT DEVELOPMENTS
20.19 FRESENIUN SE & CO
20.19.1 COMPANY OVERVIEW
20.19.2 REVENUE ANALYSIS
20.19.3 GEOGRAPHIC PRESENCE
20.19.4 PRODUCT PORTFOLIO
20.19.5 RECENT DEVELOPMENTS
20.2 NOVARTIS AG
20.20.1 COMPANY OVERVIEW
20.20.2 REVENUE ANALYSIS
20.20.3 GEOGRAPHIC PRESENCE
20.20.4 PRODUCT PORTFOLIO
20.20.5 RECENT DEVELOPMENTS
20.21 MERCK KGAA
20.21.1 COMPANY OVERVIEW
20.21.2 REVENUE ANALYSIS
20.21.3 GEOGRAPHIC PRESENCE
20.21.4 PRODUCT PORTFOLIO
20.21.5 RECENT DEVELOPMENTS
20.22 ASTRA ZENECA
20.22.1 COMPANY OVERVIEW
20.22.2 REVENUE ANALYSIS
20.22.3 GEOGRAPHIC PRESENCE
20.22.4 PRODUCT PORTFOLIO
20.22.5 RECENT DEVELOPMENTS
20.23 BRISTOL – AYERS QUIBB COMPANY
20.23.1 COMPANY OVERVIEW
20.23.2 REVENUE ANALYSIS
20.23.3 GEOGRAPHIC PRESENCE
20.23.4 PRODUCT PORTFOLIO
20.23.5 RECENT DEVELOPMENTS
20.24 ABBOTT
20.24.1 COMPANY OVERVIEW
20.24.2 REVENUE ANALYSIS
20.24.3 GEOGRAPHIC PRESENCE
20.24.4 PRODUCT PORTFOLIO
20.24.5 RECENT DEVELOPMENTS
20.25 STELLA PHARMACEUTICAL CANADA INC.
20.25.1 COMPANY OVERVIEW
20.25.2 REVENUE ANALYSIS
20.25.3 GEOGRAPHIC PRESENCE
20.25.4 PRODUCT PORTFOLIO
20.25.5 RECENT DEVELOPMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
21 RELATED REPORTS
22 CONCLUSION
23 QUESTIONNAIRE
24 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.