Global Prostate Cancer Antigen 3 Pca3 Test Market
Market Size in USD Billion
CAGR :
% 
 USD
4.43 Billion
USD
10.59 Billion
2024
2032
USD
4.43 Billion
USD
10.59 Billion
2024
2032
| 2025 –2032 | |
| USD 4.43 Billion | |
| USD 10.59 Billion | |
|
|
|
|
Prostate Cancer Antigen 3 (PCA3) Test Market Size
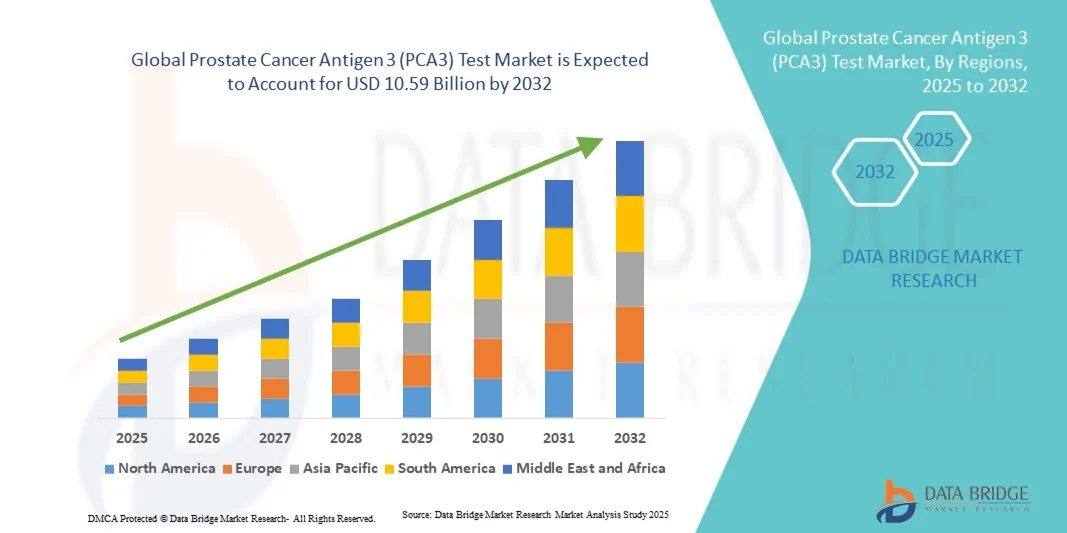
- The global Prostate Cancer Antigen 3 (PCA3) test market size was valued at USD 4.43 billion in 2024 and is expected to reach USD 10.59 billion by 2032, at a CAGR of 11.50% during the forecast period
- The market growth is largely fueled by the increasing adoption of non-invasive molecular diagnostics and the growing demand for early detection tools in prostate cancer screening, driving a shift toward precision-based testing in both clinical and diagnostic laboratory settings
- Furthermore, rising awareness of prostate cancer prevalence, combined with advancements in gene-expression analysis and integration of PCA3 testing with PSA and biopsy workflows, is establishing PCA3 as a preferred molecular marker for accurate prostate cancer assessment. These converging factors are accelerating the uptake of PCA3 test solutions, thereby significantly boosting the industry’s growth
Prostate Cancer Antigen 3 (PCA3) Test Market Analysis
- PCA3 tests, providing non-invasive molecular diagnostics for prostate cancer detection, are increasingly vital components of modern cancer screening and risk assessment in both clinical and diagnostic laboratory settings due to their enhanced accuracy, patient convenience, and ability to complement traditional PSA testing
- The escalating demand for PCA3 tests is primarily fueled by the growing adoption of precision diagnostics, rising awareness of prostate cancer prevalence, and a preference for non-invasive, urine-based molecular tests over invasive biopsy procedures
- North America dominated the PCA3 test market with the largest revenue share of 43% in 2024, characterized by early adoption of molecular diagnostic technologies, high healthcare expenditure, and a strong presence of key industry players, with the U.S. experiencing substantial growth in PCA3 testing, particularly in hospitals and private/commercial labs, driven by innovations from both established biotech firms and startups focusing on RT-PCR and other advanced assay technologies
- Asia-Pacific is expected to be the fastest-growing region in the PCA3 test market during the forecast period due to increasing healthcare infrastructure, rising awareness about prostate cancer, and expanding access to advanced diagnostic technologies
- Kits & Reagents segment dominated the PCA3 test market with a market share of 46.8% in 2024, driven by its established reputation for ease of use, compatibility with RT-PCR technology, and integration into existing molecular diagnostic workflows
Report Scope and Prostate Cancer Antigen 3 (PCA3) Test Market Segmentation
|
Attributes |
Prostate Cancer Antigen 3 (PCA3) Test Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Prostate Cancer Antigen 3 (PCA3) Test Market Trends
Advancements in Non-Invasive Urine-Based Molecular Diagnostics
- A significant and accelerating trend in the global PCA3 test market is the development and adoption of non-invasive, urine-based molecular diagnostic tests that provide accurate prostate cancer detection without the need for invasive biopsies
- For instance, the Progensa PCA3 assay allows clinicians to assess prostate cancer risk using a simple urine sample, reducing patient discomfort and streamlining the diagnostic workflow
- Integration of PCA3 testing with traditional PSA testing and other molecular markers enables more precise risk stratification and personalized patient management
- The seamless combination of PCA3 tests with laboratory information systems and automated platforms allows laboratories to increase throughput, reduce human error, and improve operational efficiency
- This trend towards more convenient, accurate, and patient-friendly molecular testing is fundamentally reshaping prostate cancer screening protocols
- The demand for non-invasive PCA3 tests is growing rapidly across hospitals, clinics, and private laboratories as physicians and patients increasingly prioritize safety, comfort, and diagnostic accuracy
- Advancements in RT-PCR technology and other molecular assay platforms are improving test sensitivity and specificity, enabling earlier and more reliable detection of prostate cancer
- Collaborations between diagnostic companies and research institutes to develop integrated prostate cancer testing panels combining PCA3 with other biomarkers are enhancing market adoption and clinical utility
Prostate Cancer Antigen 3 (PCA3) Test Market Dynamics
Driver
Increasing Awareness and Adoption of Precision Diagnostics
- The rising awareness about prostate cancer prevalence and the benefits of early detection is a significant driver for the growing adoption of PCA3 tests in clinical and diagnostic settings
- For instance, in March 2024, LabCorp expanded its prostate cancer molecular testing services, including PCA3, targeting early detection in high-risk male populations
- PCA3 tests provide enhanced diagnostic accuracy and patient convenience compared to traditional PSA tests alone, making them a preferred option for risk assessment
- Furthermore, growing adoption of precision diagnostics and personalized healthcare approaches is increasing demand for molecular tests such as PCA3, which complement standard screening protocols
- Integration with advanced laboratory workflows, electronic health records, and reporting platforms also facilitates more efficient patient management and test utilization
- The convenience of non-invasive testing, accurate risk stratification, and growing awareness among physicians and patients are key factors propelling the PCA3 test market growth globally
- Increasing government and private funding for prostate cancer research and molecular diagnostics is promoting R&D activities and accelerating PCA3 test adoption
- The rise in routine health checkups and preventive screenings, particularly in developed regions, is contributing to the steady growth of the PCA3 testing market
Restraint/Challenge
Limited Awareness and High Cost of Advanced Molecular Testing
- The relatively low awareness of PCA3 tests among certain patient populations and general practitioners poses a challenge to broader market penetration
- For instance, surveys in developing regions have shown that many physicians continue to rely primarily on PSA testing and biopsy, limiting PCA3 adoption
- High costs associated with PCA3 kits, reagents, and RT-PCR-based assays can hinder uptake in price-sensitive markets, particularly in emerging economies
- While insurance coverage and reimbursement policies are improving in developed regions, limited access and high out-of-pocket costs remain barriers in certain countries
- Addressing these challenges through physician education, awareness campaigns, and cost reduction strategies is crucial for expanding market penetration
- Overcoming limited awareness and affordability issues will be vital for sustained growth and widespread adoption of PCA3 testing in global prostate cancer screening programs
- Variability in regulatory approvals and compliance requirements across regions can delay product launch and market entry for new PCA3 tests. Lack of standardized guidelines for PCA3 test interpretation and integration into clinical decision-making may limit adoption by physicians and diagnostic labs
Prostate Cancer Antigen 3 (PCA3) Test Market Scope
The market is segmented on the basis of type of test, test type, technology, and end use.
- By Type of Test
On the basis of type of test, the PCA3 test market is segmented into instruments and kits & reagents and consumables. The Kits & Reagents segment dominated the market with the largest revenue share of 46.8% in 2024, driven by their essential role in enabling accurate PCA3 testing. These kits are widely adopted across hospitals, private laboratories, and research institutes because they provide the reagents and consumables required for sample processing and molecular analysis. High repeat usage and compatibility with standard laboratory instruments further reinforce their dominance. The segment also benefits from continuous innovation, with companies improving kit sensitivity, specificity, and ease of use, which encourages adoption in routine clinical workflows. Standardization of kits across labs ensures reliable results, contributing to their widespread preference. Moreover, their non-invasive urine-based testing compatibility makes them ideal for outpatient settings, enhancing patient compliance and clinical utility.
The Instruments segment is expected to witness the fastest growth during the forecast period due to increasing investments in laboratory infrastructure, automation, and adoption of advanced molecular diagnostic platforms. Instruments such as RT-PCR machines, automated analyzers, and microfluidic platforms are critical for running PCA3 assays efficiently. Growing awareness of precision diagnostics and increased capacity in private and public labs are driving instrument adoption. The rise of multi-parametric testing panels integrating PCA3 with other prostate cancer markers further accelerates demand for high-performance instruments. Expansion of diagnostic labs in emerging regions, coupled with government initiatives to strengthen cancer screening infrastructure, also fuels this segment’s growth.
- By Test Type
On the basis of test type, the PCA3 test market is segmented into molecular and serology. The Molecular segment dominated the market in 2024 due to the higher accuracy, reliability, and clinical acceptance of molecular tests in detecting prostate cancer at early stages. Molecular assays, including PCR-based tests, directly analyze gene expression levels, allowing precise risk stratification and reducing unnecessary biopsies. The increasing integration of molecular diagnostics into routine prostate cancer screening protocols further strengthens market dominance. Molecular tests are preferred by hospitals and high-end diagnostic labs due to their reproducibility, scalability, and compliance with clinical guidelines. Their ability to be combined with PSA and other biomarkers in multiplex panels increases clinical utility. Moreover, patient preference for non-invasive molecular testing enhances adoption in outpatient and private lab settings.
The Serology segment is expected to witness the fastest growth rate during the forecast period, driven by technological advancements enabling antibody-based detection methods for complementary cancer markers. Serology tests are cost-effective and easier to implement in smaller labs and emerging markets, where molecular infrastructure may be limited. Increasing research on combining serology with molecular tests for improved diagnostic accuracy is creating new growth opportunities. These tests also support preventive screening initiatives in community health programs, further accelerating adoption globally.
- By Technology
On the basis of technology, the PCA3 test market is segmented into RT-PCR, ELISA Test, and Micro-neutralization Assays. The RT-PCR segment dominated the market in 2024 due to its high sensitivity and specificity in detecting PCA3 gene expression levels. RT-PCR assays are widely adopted in hospitals, private labs, and research institutes for early and accurate prostate cancer diagnosis. Their compatibility with urine-based PCA3 tests and integration with automated workflows enhances operational efficiency. Continuous innovation in PCR technologies, including real-time and multiplex PCR, supports high-throughput testing and reduces turnaround time. Clinical guidelines increasingly recommend RT-PCR for PCA3 testing, driving consistent demand. Moreover, RT-PCR technology enables laboratories to integrate PCA3 testing with broader molecular diagnostic panels, increasing adoption in both developed and emerging markets.
The ELISA Test segment is expected to witness the fastest growth due to its cost-effectiveness and ease of implementation in labs with limited molecular infrastructure. ELISA-based PCA3 detection is gaining traction in emerging regions and smaller healthcare facilities where RT-PCR platforms are less accessible. Ongoing R&D is improving ELISA assay sensitivity and reproducibility, further accelerating adoption. Its scalability for routine testing and compatibility with high-throughput workflows also contribute to rapid market growth.
- By End Use
On the basis of end use, the PCA3 test market is segmented into hospitals, clinics, public health labs, private/commercial labs, physician labs, research institutes, and others. The Hospitals segment dominated the market in 2024, owing to large-scale adoption of PCA3 testing for outpatient and inpatient screening, supported by advanced laboratory infrastructure and trained personnel. Hospitals often act as early adopters of molecular diagnostics, and the ability to integrate PCA3 testing with electronic health records and clinical workflows enhances adoption. High patient volumes and routine preventive health programs in hospitals create steady demand. Hospitals also play a pivotal role in educating physicians and patients on the benefits of PCA3 tests, driving trust and market penetration. Strong collaborations with diagnostic companies for in-house testing services further reinforce hospital dominance.
The Private/Commercial Labs segment is expected to witness the fastest growth during the forecast period, driven by increasing demand for accessible, quick, and reliable PCA3 testing outside hospital settings. Expanding laboratory networks, automation, and partnerships with healthcare providers facilitate adoption. Patients increasingly prefer testing in private labs due to convenience, faster turnaround times, and confidentiality. Growth in preventive healthcare programs and corporate health initiatives also boosts demand in this segment. The availability of advanced kits, instruments, and remote sample collection services supports rapid market expansion for private and commercial labs.
Prostate Cancer Antigen 3 (PCA3) Test Market Regional Analysis
- North America dominated the PCA3 test market with the largest revenue share of 43% in 2024, characterized by early adoption of molecular diagnostic technologies, high healthcare expenditure, and a strong presence of key industry players
- Healthcare providers and patients in the region highly value the accuracy, non-invasive nature, and integration of PCA3 testing with traditional PSA screening, supporting better risk stratification and personalized treatment planning
- This widespread adoption is further reinforced by advanced healthcare infrastructure, high healthcare expenditure, strong presence of key diagnostic companies, and government initiatives promoting cancer screening, establishing PCA3 testing as a preferred solution in hospitals, clinics, and private laboratories
U.S. Prostate Cancer Antigen 3 (PCA3) Test Market Insight
The U.S. Prostate Cancer Antigen 3 (PCA3) Test Market captured the largest revenue share of 79% in 2024 within North America, fueled by the growing adoption of molecular diagnostics and increasing emphasis on early prostate cancer detection. Physicians and patients are prioritizing non-invasive, urine-based PCA3 testing to complement traditional PSA screening. The rising trend of preventive health checkups, combined with robust demand for high-accuracy diagnostic tools, further propels market growth. Moreover, integration of PCA3 testing with electronic health records and advanced laboratory workflows is significantly contributing to the market’s expansion.
Europe Prostate Cancer Antigen 3 (PCA3) Test Market Insight
The Europe Prostate Cancer Antigen 3 (PCA3) Test Market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by growing awareness of prostate cancer and stringent healthcare screening guidelines. The increase in healthcare infrastructure, coupled with rising patient preference for non-invasive diagnostics, is fostering PCA3 test adoption. European healthcare providers are also drawn to the convenience and clinical reliability these tests offer. The region is experiencing significant growth across hospitals, private labs, and clinics, with PCA3 testing being incorporated into routine cancer risk assessment protocols.
U.K. Prostate Cancer Antigen 3 (PCA3) Test Market Insight
The U.K. Prostate Cancer Antigen 3 (PCA3) Test Market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by the increasing focus on early cancer detection and personalized healthcare solutions. In addition, concerns regarding prostate cancer prevalence are encouraging hospitals, clinics, and private labs to adopt molecular diagnostic testing. The U.K.’s strong healthcare infrastructure, advanced laboratory networks, and digital health integration are expected to continue stimulating market growth.
Germany Prostate Cancer Antigen 3 (PCA3) Test Market Insight
The Germany Prostate Cancer Antigen 3 (PCA3) Test Market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing awareness of prostate cancer diagnostics and government initiatives promoting preventive health programs. Germany’s well-developed healthcare system, combined with a focus on precision medicine and clinical research, promotes PCA3 test adoption, particularly in hospitals and research institutes. Integration of PCA3 testing with molecular diagnostic workflows is also becoming increasingly prevalent, with strong demand for accurate and non-invasive testing solutions.
Asia-Pacific Prostate Cancer Antigen 3 (PCA3) Test Market Insight
The Asia-Pacific Prostate Cancer Antigen 3 (PCA3) Test Market is poised to grow at the fastest CAGR of 23% during the forecast period of 2025 to 2032, driven by increasing healthcare infrastructure, rising awareness of prostate cancer, and technological advancements in countries such as China, Japan, and India. The region's growing inclination toward preventive healthcare, supported by government initiatives promoting cancer screening, is driving PCA3 test adoption. Furthermore, as APAC emerges as a hub for molecular diagnostic development and testing, affordability and accessibility of PCA3 tests are expanding to a wider patient population.
Japan Prostate Cancer Antigen 3 (PCA3) Test Market Insight
The Japan Prostate Cancer Antigen 3 (PCA3) Test Market is gaining momentum due to the country’s advanced healthcare system, increasing focus on preventive diagnostics, and high patient awareness. The Japanese market emphasizes accuracy, convenience, and non-invasive testing, driving PCA3 test adoption in hospitals, clinics, and private labs. Integration of PCA3 testing with other molecular diagnostics for comprehensive cancer risk assessment is fueling growth. Moreover, Japan’s aging population is such asly to increase demand for efficient, patient-friendly, and reliable prostate cancer detection solutions.
India Prostate Cancer Antigen 3 (PCA3) Test Market Insight
The India Prostate Cancer Antigen 3 (PCA3) Test Market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to the expanding healthcare infrastructure, growing middle class, and rising awareness of prostate cancer. India is witnessing increasing adoption of molecular diagnostics in hospitals, clinics, and private labs. Government initiatives supporting preventive health programs and the availability of cost-effective PCA3 test kits are key factors propelling the market. The growing focus on early cancer detection and the expansion of diagnostic laboratory networks are further accelerating PCA3 test adoption across the country.
Prostate Cancer Antigen 3 (PCA3) Test Market Share
The Prostate Cancer Antigen 3 (PCA3) Test industry is primarily led by well-established companies, including:
- mdxhealth. (Belgium)
- Myriad Genetics, Inc. (U.S.)
- Abbott (U.S.)
- F. Hoffmann La Roche Ltd (Switzerland)
- Siemens Healthineers AG (Germany)
- OPKO Health, Inc. (U.S.)
- Genomic Health (U.S.)
- BD (U.S.)
- Agilent Technologies, Inc. (U.S.)
- Thermo Fisher Scientific, Inc. (U.S.)
- ExoDx (Germany)
- Creative Diagnostics (U.S.)
- Lytech (China)
- YUBO (China)
- MicroDiag Biomedicine (China)
- Bio Techne (U.S.)
- Veracyte, Inc. (U.S.)
- Danaher (U.S.)
- Beckman Coulter, Inc. (U.S.)
- BIOMÉRIEUX (France)
What are the Recent Developments in Global Prostate Cancer Antigen 3 (PCA3) Test Market?
- In February 2025, Myriad Genetics announced a strategic partnership with PATHOMIQ to licence PATHOMIQ’s AI platform for prostate cancer diagnostics and stated that they intend to commercially launch their first AI‑driven prostate cancer clinical test later in the year. Though the focus is on AI and imaging, this move is relevant to the PCA3 test space as molecular urinary diagnostics such as PCA3 are increasingly integrated with broader biomarker and AI‑driven platforms
- In February 2025, media reports highlighted a new “super‑test” developed by EDX Medical Group, which uses AI to analyse both blood and urine samples for over 100 biomarkers (including prostate cancer markers) and claims diagnostic accuracy of 96 %
- In April 2024, researchers at University of Michigan developed a next‑generation urine‑based test called MyProstateScore 2.0 (MPS2) that analyses 18 genes (including the prior PCA3 and TMPRSS2:ERG markers) and demonstrated enhanced ability to identify high‑grade prostate cancers while helping reduce unnecessary biopsies
- In April 2024, researchers funded by the National Cancer Institute (NCI) developed and externally validated a new urine‑based biomarker test that helps distinguish which men with elevated prostate‑specific antigen (PSA) should proceed to biopsy and which can safely defer. This is significant for the PCA3 test market because it underscores the growing interest and competition in non‑invasive urine assays for prostate cancer, reinforcing the utility of PCA3 and its related biomarkers in reducing unnecessary biopsies
- In March 2023, a large‑scale prospective multicenter study conducted in China involving 1,117 patients evaluated urinary PCA3 assay performance and found that men with positive biopsy results had significantly higher median PCA3 scores than those with negative biopsies strengthening the marker’s utility across different populations
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.