Global Protac Targeted Protein Degrader Therapies Market
Market Size in USD Billion
CAGR :
% 
 USD
1.12 Billion
USD
6.30 Billion
2025
2033
USD
1.12 Billion
USD
6.30 Billion
2025
2033
| 2026 –2033 | |
| USD 1.12 Billion | |
| USD 6.30 Billion | |
|
|
|
|
PROTAC Targeted Protein Degrader Therapies Market Size
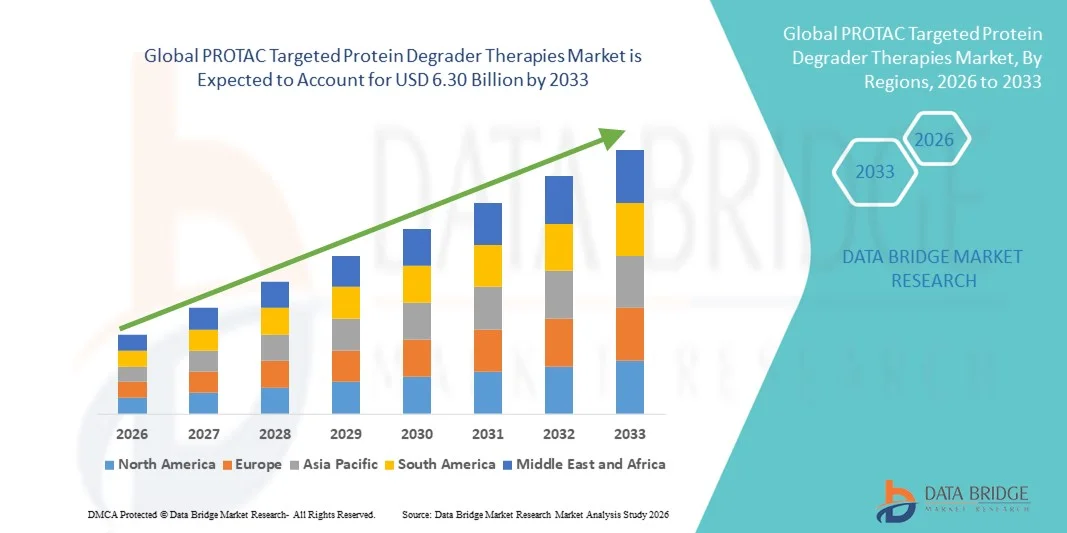
- The global PROTAC targeted protein degrader therapies market size was valued at USD 1.12 billion in 2025 and is expected to reach USD 6.30 billion by 2033, at a CAGR of 24.10% during the forecast period
- The market growth is largely fueled by rapid advancements in targeted protein degradation technologies, growing research investments, and increasing adoption of precision medicine approaches in drug development
- Furthermore, rising demand for novel therapies to address oncology, neurodegenerative, and autoimmune diseases is driving the development and uptake of PROTAC Targeted Protein Degrader Therapies, thereby significantly boosting the industry’s growth
PROTAC Targeted Protein Degrader Therapies Market Analysis
- PROTAC Targeted Protein Degrader Therapies, offering selective degradation of disease-causing proteins, are increasingly vital components of modern drug discovery and therapeutic strategies in oncology, neurodegenerative disorders, and inflammatory diseases due to their high specificity, reduced off-target effects, and potential to target previously “undruggable” proteins
- The escalating demand for PROTAC therapies is primarily fueled by the growing adoption of targeted therapies, increasing investment in biotechnology research, and a rising preference for precision medicine approaches in difficult-to-treat diseases
- North America dominated the PROTAC Targeted Protein Degrader Therapies market with the largest revenue share of 42% in 2025, characterized by advanced R&D infrastructure, strong presence of key biotech and pharmaceutical players, and early clinical adoption, with the U.S. experiencing substantial growth in PROTAC therapy development and application, particularly in oncology and neurodegenerative disease research
- Asia-Pacific is expected to be the fastest-growing region in the PROTAC Targeted Protein Degrader Therapies market during the forecast period with a share of 28% by 2030, due to increasing biotech investments, expanding clinical research infrastructure, and rising healthcare expenditure in countries such as China, Japan, and India
- The Oncology segment dominated the largest market revenue share of 57.1% in 2025, owing to high prevalence of cancers, established clinical evidence, and robust adoption of protein degradation strategies
Report Scope and PROTAC Targeted Protein Degrader Therapies Market Segmentation
|
Attributes |
PROTAC Targeted Protein Degrader Therapies Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
PROTAC Targeted Protein Degrader Therapies Market Trends
Enhanced Convenience Through Advanced Therapeutic Developments
- A significant and accelerating trend in the global PROTAC Targeted Protein Degrader Therapies market is the increasing focus on developing targeted protein degraders with higher selectivity, improved pharmacokinetics, and lower off-target toxicity
- This trend is enhancing therapeutic efficacy and patient outcomes across oncology and other disease areas
- For instance, in May 2025, Arvinas announced the advancement of its ARV-471 candidate in clinical trials for breast cancer, demonstrating improved protein degradation activity and tolerability compared with standard therapies. Similarly, Kymera Therapeutics reported preclinical success with its IRAK4-targeting degrader, highlighting potential for autoimmune disease treatment
- The trend towards multi-targeted PROTAC molecules is enabling the simultaneous degradation of disease-relevant proteins, creating new opportunities for combination therapies and expanding the range of treatable conditions
- The growing investment in personalized medicine and companion diagnostics is accelerating the design of PROTACs tailored to specific patient populations, increasing the precision and efficacy of these therapies
- Regulatory incentives and fast-track approvals in key markets are supporting the rapid development and adoption of PROTAC therapies, fostering collaborations between biotech startups and established pharmaceutical companies
- This trend is reshaping treatment paradigms, particularly in oncology, by offering alternative therapeutic options for previously undruggable targets, and is expected to continue driving innovation and investment in the sector
PROTAC Targeted Protein Degrader Therapies Market Dynamics
Driver
Rising Demand for Targeted Therapies and Improved Patient Outcomes
- The increasing prevalence of cancers and chronic diseases, along with the limitations of traditional small-molecule inhibitors, is driving demand for PROTAC-based therapies with improved specificity and reduced side effects
- For instance, in June 2024, Pfizer announced collaboration with a leading biotech company to advance PROTAC candidates targeting hematologic malignancies, highlighting industry confidence in targeted protein degradation approaches
- Growing research investments, supportive funding initiatives, and a surge in biotech startups focusing on PROTACs are further accelerating pipeline expansion
- The desire for therapies that overcome drug resistance mechanisms and degrade previously undruggable proteins is creating a strong pull for innovative PROTAC molecules
- Increasing adoption of clinical trials across North America, Europe, and Asia-Pacific for both oncology and non-oncology indications is expanding the evidence base and supporting commercialization strategies
Restraint/Challenge
High Development Costs and Regulatory Complexities
- The complex design, synthesis, and optimization of PROTAC molecules require substantial R&D investment, posing a barrier for smaller biotech firms and impacting overall market growth
- For instance, in February 2025, a mid-sized biotech company reported delays in clinical development due to high costs associated with preclinical pharmacology and toxicology studies of its PROTAC candidates
- Regulatory uncertainty regarding safety evaluation, long-term effects, and first-in-class approval pathways may slow market entry and commercialization timelines
- Challenges in large-scale manufacturing, stability, and formulation of PROTAC compounds remain critical hurdles that require technical innovation and collaboration with contract manufacturing organizations
- While potential efficacy and patient benefits are high, pricing pressures and reimbursement uncertainties may restrict adoption, particularly in cost-sensitive healthcare systems
- Addressing these challenges through strategic partnerships, robust clinical evidence generation, and streamlined regulatory guidance will be essential for sustained growth in the PROTAC Targeted Protein Degrader Therapies market
PROTAC Targeted Protein Degrader Therapies Market Scope
The market is segmented on the basis of type, mechanism of action, and application.
- By Type
On the basis of type, the PROTAC Targeted Protein Degrader Therapies market is segmented into Small Molecule PROTACs, Peptide-based PROTACs, and Other PROTAC Modalities. The Small Molecule PROTACs segment dominated the largest market revenue share of 48.3% in 2025, driven by its high bioavailability, well-characterized pharmacokinetics, and strong efficacy in oncology applications. These PROTACs are widely preferred in preclinical and clinical research due to established chemical synthesis protocols. Small molecule PROTACs demonstrate excellent cell permeability and systemic distribution, supporting consistent therapeutic outcomes. Hospitals and research centers favor these molecules for targeted protein degradation studies. Their versatility allows application across multiple disease areas. Regulatory approvals and ongoing clinical trials reinforce market confidence. High adoption in both developed and emerging markets sustains revenue growth. Integration with combinatorial therapies enhances efficacy. Drug pipeline expansion by key pharmaceutical companies further strengthens market leadership. Strong patent portfolios protect market share. Widespread familiarity among researchers ensures continuous preference. Overall, these factors secured the dominant position of Small Molecule PROTACs in 2025.
The Peptide-based PROTACs segment is expected to witness the fastest CAGR of 14.2% from 2026 to 2033, fueled by advances in peptide stability, cell penetration techniques, and targeted delivery systems. Peptide PROTACs offer precise protein recognition, making them attractive for neurodegenerative and autoimmune disease research. Rising investment in peptide therapeutics by biotech firms accelerates adoption. Improved conjugation technologies enhance efficacy and reduce off-target effects. Growing preclinical data supporting peptide PROTAC safety drives confidence among clinical researchers. Increasing demand for personalized medicine applications boosts growth. Integration with nanoparticle and carrier systems supports enhanced delivery. Expanding collaborations between academia and industry foster innovation. Emerging market interest further accelerates revenue potential. Rapid pipeline expansion in oncology and immunology strengthens uptake. Supportive regulatory pathways for experimental therapeutics enhance accessibility. Overall, these advantages position peptide-based PROTACs as the fastest-growing type segment.
- By Mechanism of Action
On the basis of mechanism of action, the market is segmented into Ubiquitin-Proteasome Pathway, Autophagy-Lysosome Pathway, and Other Degradation Pathways. The Ubiquitin-Proteasome Pathway segment dominated with 52.7% revenue share in 2025, driven by its well-established mechanism for selective protein degradation. This pathway has strong clinical validation, particularly in oncology, enabling robust therapeutic outcomes. Hospitals and research institutes rely heavily on ubiquitin-based PROTACs due to predictable pharmacodynamics. Availability of standardized reagents supports consistent research results. High adoption in preclinical pipelines strengthens market dominance. Favorable patent protection ensures market exclusivity for key players. Efficient cellular clearance minimizes off-target toxicity. Integration into combination therapies enhances efficacy in resistant cancers. Ongoing clinical trials continue to expand indications. Reimbursement frameworks in advanced markets support therapeutic utilization. Multi-disease applicability further increases demand. Overall, these factors secured leadership in 2025.
The Autophagy-Lysosome Pathway segment is projected to register the fastest CAGR of 13.5% from 2026 to 2033, driven by rising research interest in neurodegenerative and metabolic disorders. This pathway enables degradation of aggregated proteins, addressing previously “undruggable” targets. Technological advances in lysosome-targeting chimeras (LYTACs) strengthen clinical translation. Academic and biotech collaborations accelerate pipeline growth. Growing preclinical evidence demonstrates efficacy and safety, boosting adoption. Emerging therapies targeting autophagy modulation expand clinical relevance. Rising funding for rare disease research fuels market expansion. Enhanced understanding of cellular trafficking improves therapeutic design. Market interest is increasing in Asia-Pacific and North America. Expansion of regulatory support for experimental therapeutics encourages growth. Overall, these factors make autophagy-based PROTACs the fastest-growing mechanism segment.
- By Application
On the basis of application, the market is segmented into Oncology, Neurodegenerative Diseases, Inflammatory & Autoimmune Diseases, and Others. The Oncology segment dominated the largest market revenue share of 57.1% in 2025, owing to high prevalence of cancers, established clinical evidence, and robust adoption of protein degradation strategies. Hospitals and specialty clinics are increasingly incorporating PROTACs for solid and hematological tumors. Combination therapy strategies with kinase inhibitors or immunotherapies enhance clinical outcomes. Oncology-focused R&D investments by pharmaceutical companies continue to expand pipelines. Advanced clinical trial networks support rapid patient recruitment. Regulatory approvals and fast-track designations favor oncology PROTACs. Collaboration between academia and industry drives innovation. Strong patent protections sustain market leadership. Established dosing protocols and familiarity among oncologists reinforce adoption. Global prevalence of targetable oncoproteins maintains consistent demand. Overall, oncology PROTACs remain the dominant application segment.
The Neurodegenerative Diseases segment is expected to witness the fastest CAGR of 12.8% from 2026 to 2033, driven by increasing incidence of Alzheimer’s, Parkinson’s, and related disorders. Rising research focus on aggregated protein degradation and synaptic protection fuels pipeline expansion. Hospitals and specialized neurology centers adopt targeted therapies for experimental clinical trials. Integration with biomarker-driven patient stratification supports precise interventions. Government and private funding for neurodegenerative research accelerates development. Improved CNS delivery strategies enhance therapeutic potential. Positive preclinical outcomes bolster confidence in translation to human studies. Biotech investments in peptide-based and lysosome-targeting PROTACs drive growth. Personalized medicine trends increase demand. Adoption in emerging markets further contributes to expansion. Overall, these factors position neurodegenerative applications as the fastest-growing segment.
PROTAC Targeted Protein Degrader Therapies Market Regional Analysis
- North America dominated the PROTAC Targeted Protein Degrader Therapies market with the largest revenue share of 42% in 2025, characterized by advanced R&D infrastructure, a strong presence of key biotech and pharmaceutical players, and early clinical adoption of targeted protein degrader therapies. The U.S. experienced substantial growth in PROTAC therapy development and application, particularly in oncology and neurodegenerative disease research, supported by robust clinical trial activity, regulatory engagement, and strategic collaborations among global drug developers
- Consumers and healthcare stakeholders in the region increasingly value the potential of PROTACs to degrade previously “undruggable” targets, offering new treatment paradigms for hard‑to‑treat cancers and chronic diseases
- This adoption is further supported by high healthcare R&D spending, well‑established clinical networks, and strong investment in precision medicine
U.S. PROTAC Targeted Protein Degrader Therapies Market Insight
The U.S. PROTAC targeted protein degrader therapies market captured the largest revenue share within North America in 2025, driven by rapid advancements in PROTAC drug candidates and strong clinical pipeline progression in oncology and neurodegenerative indications. The growing number of partnerships between biotech innovators and large pharmaceutical companies, coupled with supportive regulatory pathways (including Fast Track and Breakthrough Therapy designations), is accelerating commercialization and adoption of PROTAC therapies. Furthermore, escalating investment in clinical research infrastructure and biotechnology clusters in states such as Massachusetts and California is significantly contributing to market expansion.
Europe PROTAC Targeted Protein Degrader Therapies Market Insight
The Europe PROTAC targeted protein degrader therapies market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by increasing clinical research initiatives, regulatory harmonization for innovative biologics, and expanding oncology research programs across the EU. European countries are witnessing significant academic and industry collaborations aimed at advancing PROTAC platforms, particularly for cancer and neurodegenerative disorders. Growing healthcare expenditure and focus on precision medicine are supporting wider clinical adoption of targeted degrader therapies across key European markets.
U.K. PROTAC Targeted Protein Degrader Therapies Market Insight
The U.K. PROTAC targeted protein degrader therapies market is anticipated to grow at a noteworthy CAGR during the forecast period, fueled by the country’s strong biomedical research ecosystem, established drug discovery infrastructure, and active participation in global clinical trials assessing PROTAC candidates. Additionally, strategic investments by government and private research entities are fostering innovation in targeted protein degradation, especially for oncology and CNS indications, further stimulating market growth.
Germany PROTAC Targeted Protein Degrader Therapies Market Insight
The Germany PROTAC targeted protein degrader therapies market is expected to expand at a considerable CAGR during the forecast period, driven by the nation’s well‑developed healthcare and life sciences sector, robust research and development base, and strong presence of contract research organizations (CROs) supporting PROTAC development and translational research. Germany’s emphasis on innovation and access to cutting‑edge therapeutic platforms promotes adoption of PROTAC therapies, particularly in academic and industrial drug discovery settings.
Asia‑Pacific PROTAC Targeted Protein Degrader Therapies Market Insight
The Asia‑Pacific PROTAC targeted protein degrader therapies market is expected to be the fastest‑growing region during the forecast period, with a projected share of 28% by 2030, due to increasing biotech investments, expanding clinical research infrastructure, and rising healthcare expenditure in countries such as China, Japan, and India. Governments and private sectors are intensifying efforts to build biopharmaceutical innovation hubs, attract international clinical trials, and support translational research in novel therapeutic modalities like PROTACs.
Japan PROTAC Targeted Protein Degrader Therapies Market Insight
The Japan PROTAC targeted protein degrader therapies market is gaining momentum due to the country’s strong scientific research base, high healthcare spending, and focused investment in precision medicine and next‑generation biologics. The launch of local clinical trials and collaborations between Japanese biotech firms and global developers are contributing to accelerated PROTAC research and prospective treatment options for oncology and neurodegenerative diseases.
China PROTAC Targeted Protein Degrader Therapies Market Insight
The China PROTAC targeted protein degrader therapies market accounted for a significant share in Asia‑Pacific in 2025, attributed to the country’s expanding biotech ecosystem, strong domestic pharmaceutical manufacturing capabilities, and increasing clinical R&D initiatives. China is emerging as a key hub for PROTAC drug discovery and early‑phase clinical testing, supported by rising healthcare investments and an expanding pool of scientific talent, which are driving broader market adoption and innovation across therapeutic areas such as cancer and inflammatory diseases.
PROTAC Targeted Protein Degrader Therapies Market Share
The PROTAC Targeted Protein Degrader Therapies industry is primarily led by well-established companies, including:
- Arvinas (U.S.)
- C4 Therapeutics (U.S.)
- Nurix Therapeutics (U.S.)
- Kymera Therapeutics (U.S.)
- Vividion Therapeutics (U.S.)
- Foghorn Therapeutics (U.S.)
- Amphista Therapeutics (U.K.)
- ProteoChem (Germany)
- Prothena Corporation (Ireland)
- Monte Rosa Therapeutics (Switzerland)
- Plexium (Canada)
- Frontier Medicines (China)
- Ascendia Pharmaceuticals (Japan)
- X-Chem (U.S.)
- OncoArendi Therapeutics (Poland)
- Heptares Therapeutics (U.K.)
- Evotec (Germany)
- BioTheryX (U.S.)
- Kyowa Kirin (Japan)
- ORIC Pharmaceuticals (U.S.)
Latest Developments in Global PROTAC Targeted Protein Degrader Therapies Market
- In February 2024, Arvinas announced first‑in‑human dosing of ARV‑102, its first oral PROTAC protein degrader designed to cross the blood‑brain barrier and target LRRK2 for the treatment of neurodegenerative diseases. The Phase 1 trial evaluated safety, tolerability, pharmacokinetics, and pharmacodynamics in healthy volunteers, expanding PROTAC applications beyond oncology to neurological targets
- In April 2024, Arvinas and Novartis entered an exclusive global licensing agreement for ARV‑766, an orally bioavailable PROTAC that selectively targets and degrades the androgen receptor for metastatic prostate cancer. Under the agreement, Novartis acquired Arvinas’ preclinical AR‑V7 program as part of broader collaboration to develop next‑generation targeted degraders
- In February 2024, the U.S. FDA granted Fast Track designation to Vepdegestrant (ARV‑471), an oral estrogen receptor‑targeted PROTAC in development for adults with ER+/HER2‑ advanced or metastatic breast cancer, signaling regulatory support for accelerated development of PROTAC degraders in oncology
- In April 2025, BeiGene launched the first Phase III clinical trial of BGB‑16673, a BTK‑targeting PROTAC for patients with chronic lymphocytic leukemia (CLL) who previously received both BTK and BCL‑2 inhibitors, marking one of the earliest degraders to reach late‑stage clinical evaluation in hematologic malignancies
- In June 2025, Arvinas and Pfizer submitted a New Drug Application (NDA) to the U.S. FDA for Vepdegestrant (ARV‑471), seeking approval for this oral PROTAC therapy to treat ESR1‑mutated ER+/HER2‑ advanced breast cancer. If approved, this would represent the world’s first commercial PROTAC degrader therapy
- In March 2025, clinical updates reported that ARV‑471 continued to demonstrate encouraging clinical benefits in Phase 3 studies, with investors and researchers highlighting its potential to outperform existing therapies in ER+/HER2‑ metastatic breast cancer settings
- In April 2025, research landscapes showed that PROTAC R&D significantly expanded, with over 90 targeted degrader candidates in clinical development and strategic collaborations among pharma companies advancing the modality in oncology and autoimmune indications
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.