Global Protein Stability Analysis Market
Market Size in USD Billion
CAGR :
% 
 USD
1.42 Billion
USD
2.68 Billion
2024
2032
USD
1.42 Billion
USD
2.68 Billion
2024
2032
| 2025 –2032 | |
| USD 1.42 Billion | |
| USD 2.68 Billion | |
|
|
|
|
Protein Stability Analysis Market Size
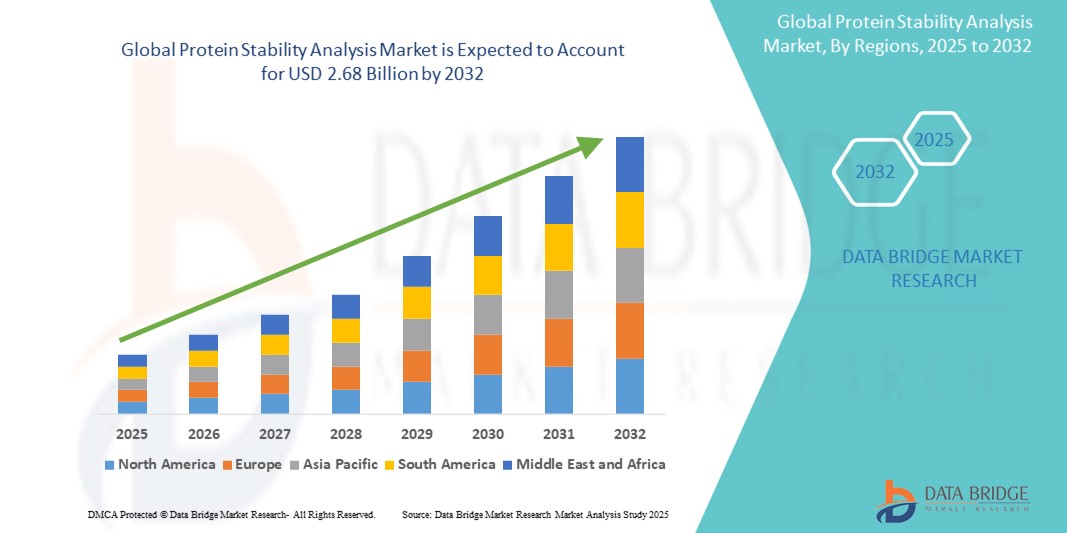
- The global protein stability analysis market size was valued at USD 1.42 billion in 2024 and is expected to reach USD 2.68 billion by 2032, at a CAGR of 8.22% during the forecast period
- The market growth for protein stability analysis is largely fuelled by the escalating demand for biopharmaceuticals and protein-based therapeutics, along with continuous technological advancements in analytical instruments and assays, leading to increased focus on quality control and regulatory compliance in the biotechnology and pharmaceutical sectors
- Furthermore, rising R&D investments in drug discovery and development, coupled with a growing emphasis on understanding protein behaviour for improved drug formulations and efficacy, is establishing protein stability analysis as a critical step in the biopharmaceutical lifecycle. These converging factors are accelerating the uptake of protein stability analysis solutions, thereby significantly boosting the industry's growth
Protein Stability Analysis Market Analysis
- Protein stability analysis, which involves assessing the structural integrity and functional activity of proteins over time, is an increasingly vital process in biopharmaceutical development and quality control. This is due to its critical role in ensuring the safety, efficacy, and shelf-life of protein-based therapeutics and biologics across various research and industrial settings
- The escalating demand for protein stability analysis is primarily fueled by the rapid growth of the biopharmaceutical industry, the increasing complexity of protein therapeutics, and stringent regulatory requirements for drug characterization and formulation. This is establishing protein stability analysis as a fundamental and indispensable step in the drug development pipeline
- North America dominates the protein stability analysis market, with the largest revenue share of 49.3% in 2024, characterized by a high concentration of major pharmaceutical and biotechnology companies, substantial R&D investments, and a robust regulatory framework that mandates rigorous protein characterization
- Asia-Pacific is expected to be the fastest-growing region in the protein stability analysis market during the forecast period, with a CAGR of 12.15%. This rapid growth is primarily due to the expansion of pharmaceutical manufacturing capabilities, increasing R&D activities in biosimilars and biologics, and improving healthcare infrastructure in countries such as China, India, and South Korea
- The reagents and assay kits segment dominates the protein stability analysis market, market share of 44.3% in 2024. This is driven by their continuous consumption in routine stability testing, their crucial role in various analytical techniques, and the ongoing development of specialized kits for specific protein types and stability challenges
Report Scope and Protein Stability Analysis Market Segmentation
|
Attributes |
Protein Stability Analysis Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Protein Stability Analysis Market Trends
“Automated High-Throughput Analysis and Predictive Modeling”
- A significant and accelerating trend in the global protein stability analysis market is the deepening integration of advanced automation, robotics, and artificial intelligence (AI) to enhance the throughput, precision, and predictive capabilities of stability studies. This fusion of technologies is fundamentally transforming how biopharmaceutical companies analyze and optimize protein formulations
- For instance, automated liquid handling systems and robotic platforms are routinely used to prepare and analyze hundreds or thousands of protein samples in parallel, enabling rapid screening of various formulation conditions. Similarly, machine learning algorithms are being developed and applied to predict protein stability based on sequence, structure, and experimental data, significantly accelerating the early stages of drug development
- AI integration in protein stability analysis enables features such as learning optimal assay parameters, predicting the long-term stability of a protein based on short-term experimental data, and identifying critical degradation pathways. For instance, some advanced analytical platforms utilize AI to interpret complex spectroscopic data, improving the accuracy of aggregation detection, or can flag unusual stability profiles for further investigation. Furthermore, automation capabilities offer users the ease of hands-free operation, allowing scientists to conduct complex experiments with minimal manual intervention, freeing up time for data interpretation and strategic decision-up decisions
- The seamless integration of protein stability analysis instruments with laboratory information management systems (LIMS) and broader R&D data platforms facilitates centralized control and data management across various stages of biopharmaceutical development. Through a single interface, users can manage experimental protocols, track sample progress, and analyze stability data alongside other characterization parameters, creating a unified and automated R&D workflow
- This trend towards more intelligent, intuitive, and interconnected analytical systems is fundamentally reshaping user expectations for protein characterization. Consequently, companies are developing AI-enabled analytical instruments and software solutions with features such as automated data interpretation, predictive modeling for shelf-life, and integrated regulatory compliance reporting
- The demand for protein stability analysis solutions that offer seamless automation and AI-driven insights is growing rapidly across pharmaceutical, biotechnology, and contract research organizations, as these industries increasingly prioritize efficiency, speed-to-market, and robust data integrity for their protein therapeutics
Protein Stability Analysis Market Dynamics
Driver
“Growing Need Due to Biopharmaceutical Industry Expansion and Regulatory Mandates”
- The rapid expansion of the biopharmaceutical industry, particularly the increasing pipeline of complex protein-based therapeutics and biosimilars, coupled with stringent regulatory requirements for drug approval and quality control, is a significant driver for the heightened demand for protein stability analysis
- For instance, in 2024, leading biopharmaceutical companies continued to invest heavily in novel monoclonal antibodies and gene therapies, for which robust stability data are paramount to ensure safety and efficacy throughout their shelf-life. Such strategies by key industry players are expected to drive the protein stability analysis industry growth in the forecast period
- As developers become more aware of the inherent instability of proteins and seek to ensure the integrity and longevity of their therapeutic products, protein stability analysis offers advanced insights into degradation pathways, aggregation propensities, and conformational changes, providing a compelling upgrade over basic characterization methods
- Furthermore, the growing global demand for high-quality biopharmaceuticals and the desire for extended product shelf-lives are making protein stability analysis an integral component of drug development, offering seamless integration with formulation development, manufacturing processes, and quality assurance protocols
- The convenience of early-stage screening for stable candidates, the ability to optimize formulations to prevent aggregation and degradation, and the necessity to meet rigorous regulatory guidelines are key factors propelling the adoption of protein stability analysis in both pharmaceutical and biotechnology sectors. The trend towards accelerated stability testing and the increasing availability of sophisticated analytical platforms further contribute to market growth
Restraint/Challenge
“Concerns Regarding High Instrumentation Costs and Data Interpretation Complexity”
- Concerns surrounding the substantial initial investment required for advanced protein stability analysis instrumentation, coupled with the inherent complexity of interpreting heterogeneous protein degradation data, pose a significant challenge to broader market penetration. As protein stability analysis relies on highly specialized and sensitive analytical techniques, these factors raise anxieties among potential adopters about the economic feasibility and expertise required
- For instance, high-profile reports on the capital outlay for state-of-the-art spectroscopy or calorimetry instruments have made some smaller biotechnology firms or academic labs hesitant to fully equip their stability testing capabilities
- Addressing these cost concerns through the development of more affordable, versatile instruments, alongside robust data analysis software and standardized methodologies, is crucial for building broader adoption. Companies such as Waters and Malvern Panalytical are emphasizing the cost-effectiveness and user-friendliness of their integrated solutions in their marketing to reassure potential buyers. In addition, the relatively high complexity of protein structures and their multiple degradation pathways can lead to challenges in data interpretation, requiring highly skilled personnel. While analytical methods are advancing, deriving clear, actionable insights from complex stability profiles remains a bottleneck for some users
- While prices for some technologies are gradually decreasing, the perceived premium for high-end analytical equipment can still hinder widespread adoption, especially for organizations with limited budgets or those lacking in-house expertise for advanced protein characterization
- Overcoming these challenges through enhanced accessibility of analytical services, improved automation for simplified operation, and the development of more intuitive data analysis tools will be vital for sustained market growth
Protein Stability Analysis Market Scope
The protein stability analysis market is segmented into three notable segments based on Product, Technique, and End Use.
- By Product
On the basis of product, the protein stability analysis market is segmented into reagents and assay kits, instruments, consumables and accessories, and software. The reagents and assay kits segment held the largest market revenue share 44.3% in 2024. This is driven by their continuous and high-volume consumption in routine stability testing, their crucial role in various analytical techniques, and the ongoing development of specialized kits for specific protein types and stability challenges. The frequent repurchase cycle of these consumables contributes significantly to their market presence.
The software segment is anticipated to witness a high growth rate from 2025 to 2032, fueled by the increasing need for advanced data analysis, automation of experimental workflows, and integration with laboratory information management systems (LIMS). As data complexity grows, sophisticated software solutions become indispensable for interpreting results, predicting stability, and ensuring regulatory compliance.
- By Technique
On the basis of technique, the protein stability analysis market is segmented into chromatography, spectroscopy, surface plasmon resonance imaging (spri), differential scanning calorimetry (DSC), differential scanning fluorimetry (DSF), and others. The chromatography segment held the market revenue share in 2024, driven by its established reputation for high-resolution separation and precise quantification of protein variants and aggregates. Techniques such as high-performance liquid chromatography (HPLC) and size-exclusion chromatography (SEC) are fundamental for assessing protein purity and identifying degradation products.
The differential scanning fluorimetry (DSF) segment is expected to witness a rapid growth rate from 2025 to 2032. This is favored for its high-throughput capabilities, minimal sample consumption, and ability to quickly determine protein melting temperatures (Tm), making it ideal for early-stage screening in drug discovery and formulation development. DSF offers a convenient and rapid method for assessing the thermal stability of a large number of samples, driving its increasing adoption.
- By End Use
On the basis of end use, the protein stability analysis market is segmented into pharmaceutical and biotechnology companies, contract research organizations (CROs), and academic and research institutes. The pharmaceutical and biotechnology companies segment held the largest market share of 52.3% in 2024. This is driven by the vast number of protein-based drug candidates in development, stringent regulatory requirements for stability testing throughout the drug lifecycle, and significant in-house R&D investments by these companies.
The contract research organizations (CROs) segment is expected to witness a robust growth rate from 2025 to 2032. This growth is fueled by the increasing trend of outsourcing specialized analytical services by pharmaceutical and biotechnology companies, particularly smaller firms or those seeking to leverage CROs' expertise and advanced instrumentation without incurring high capital costs. CROs offer a cost-effective and efficient solution for comprehensive protein stability analysis.
Protein Stability Analysis Market Regional Analysis
- North America dominates the Protein Stability Analysis market with the largest revenue share, estimated to be around 49.3% in 2024. This leadership is driven by the region's robust biopharmaceutical industry, significant investments in R&D, and the presence of numerous major pharmaceutical and biotechnology companies. The strong emphasis on innovative drug development and stringent regulatory frameworks for protein therapeutics particularly in the U.S. and Canada, fuel the demand for comprehensive protein stability analysis solutions
- Companies and research institutions in the region highly value the precision, high-throughput capabilities, and advanced data interpretation offered by cutting-edge protein stability analysis instruments and services. This widespread adoption is further supported by high disposable incomes (relative to R&D budgets), a technologically advanced scientific community, and the growing preference for advanced analytical techniques to ensure drug safety, efficacy, and longer shelf life
- This widespread adoption is further supported by substantial funding from public and private entities for proteomics and biopharmaceutical research, a technologically advanced scientific community, and the growing focus on developing complex biologics and biosimilars. These factors firmly establish protein stability analysis as a favored and indispensable solution for both preclinical and clinical stages of drug development in North America
U.S. Protein Stability Analysis Market Insight
The U.S. protein stability analysis market captured largest revenue share of 76.8% in 2024 within North America. This dominance is fueled by the swift expansion of the biopharmaceutical sector, the presence of numerous global pharmaceutical and biotechnology giants, and substantial R&D investments in novel protein therapeutics. The country's robust regulatory environment, which mandates extensive stability testing for drug approval, further propels the protein stability analysis industry. Moreover, the increasing integration of advanced analytical technologies, such as high-throughput screening and AI-driven data analysis, is significantly contributing to the market's expansion, driven by the need for faster drug development cycles.
Europe Protein Stability Analysis Market Insight
The Europe protein stability analysis market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by strong biopharmaceutical R&D activities, particularly in countries such as Germany, Switzerland, and the UK, and the escalating need for rigorous quality control in drug manufacturing. The increase in regulatory scrutiny by agencies such as the EMA, coupled with rising investments in biologics and biosimilars, is fostering the adoption of advanced protein stability analysis techniques. European consumers are also benefiting from the availability of a wide range of protein-based therapies, which necessitates robust stability assessment throughout their lifecycle. The region is experiencing significant growth across pharmaceutical, biotechnology, and academic research applications, with protein stability analysis being incorporated into both early-stage discovery and late-stage formulation development.
U.K. Protein Stability Analysis Market Insight
The U.K. protein stability analysis market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by a thriving biotechnology sector and a desire for enhanced drug efficacy and safety. In addition, the country's strong academic research base and increasing funding for life sciences are encouraging both pharmaceutical companies and research institutes to invest in advanced protein stability analysis solutions. The UK’s focus on fostering innovation in drug development, alongside its well-established regulatory infrastructure, is expected to continue to stimulate market growth.
Germany Protein Stability Analysis Market Insight
The Germany protein stability analysis market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing awareness of biopharmaceutical quality standards and the demand for technologically advanced, high-precision analytical solutions. Germany’s well-developed pharmaceutical manufacturing infrastructure, combined with its emphasis on innovation and robust scientific research, promotes the adoption of protein stability analysis, particularly in bioprocessing and quality assurance departments. The integration of protein stability analysis with automated systems is also becoming increasingly prevalent, with a strong preference for efficient, data-driven solutions aligning with local industry expectations.
Asia-Pacific Protein Stability Analysis Market Insight
The Asia-Pacific protein stability analysis market is poised to grow at the fastest CAGR of 12.15% during the forecast period of 2025 to 2032. This rapid growth is driven by increasing R&D investments in biopharmaceuticals, rising disposable incomes leading to better healthcare access, and technological advancements in countries such as China, Japan, and India. The region's growing inclination towards domestic drug development and manufacturing, supported by government initiatives promoting biotech industries, is driving the adoption of protein stability analysis. Furthermore, as APAC emerges as a manufacturing hub for biologics and biosimilars, the need for robust and efficient protein stability analysis is expanding to a wider range of companies.
Japan Protein Stability Analysis Market Insight
The Japan protein stability analysis market is gaining momentum due to the country’s high-tech scientific culture, rapid advancements in biotechnology, and demand for high-quality pharmaceutical products. The Japanese market places a significant emphasis on drug safety and efficacy, and the adoption of protein stability analysis is driven by the increasing number of biologics in development and the rigorous regulatory environment. The integration of protein stability analysis with other biophysical characterization techniques and automated platforms is fueling growth. Moreover, Japan's commitment to cutting-edge medical research and its strong pharmaceutical industry are likely to spur demand for sophisticated protein stability analysis solutions.
China Protein Stability Analysis Market Insight
The China protein stability analysis market accounted for a substantial market revenue share in Asia Pacific in 2024, attributed to the country's expanding biopharmaceutical industry, rapid growth in R&D capabilities, and high rates of technological adoption. China stands as one of the largest emerging markets for biologics and biosimilars, and protein stability analysis is becoming increasingly crucial in domestic drug development and manufacturing. The push towards establishing a leading position in global biopharma and the availability of increasingly sophisticated protein stability analysis options, alongside strong domestic manufacturers and service providers, are key factors propelling the market in China..
Protein Stability Analysis Market Share
The protein stability analysis industry is primarily led by well-established companies, including:
- Unchained Labs (U.S.)
- ProtaGene Group (Germany)
- Charles River Laboratories (U.S.)
- Intas Pharmaceuticals Ltd. (India)
- Amgen Inc. (U.S.)
- Sartorius AG (Germany)
- Neurelis, Inc. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- Agilent Technologies, Inc. (U.S.)
- PerkinElmer (U.S.)
- Waters Corporation (U.S.)
- General Electric Company (U.S.)
- Horiba (Japan)
- Spectris (U.K.)
- Enzo Biochem Inc. (U.S.)
- KEP TECHNOLOGIES (France)
Latest Developments in Global Protein Stability Analysis Market
- In April 2023, Waters Corporation continued its strategic initiatives aimed at enhancing biopharmaceutical characterization, including protein stability analysis, through advancements in its chromatography and mass spectrometry platforms. This underscores the company's dedication to delivering innovative, reliable analytical solutions tailored to the rigorous demands of biologics development. By leveraging its global expertise and cutting-edge product offerings, Waters is not only addressing the increasing complexity of protein therapeutics but also reinforcing its position in the rapidly growing global protein stability analysis market
- In March 2023, Unchained Labs, a prominent company specializing in protein characterization, introduced new software functionalities for its high-throughput analytical platforms. These advancements were specifically engineered to streamline data analysis for protein stability studies, enhancing efficiency for biopharmaceutical researchers. The innovative updates are designed to improve data interpretation and accelerate the formulation development process, highlighting Unchained Labs' commitment to developing cutting-edge technologies that safeguard the integrity and efficacy of protein therapeutics
- In March 2023, Thermo Fisher Scientific Inc. successfully launched new integrated solutions aimed at enhancing the workflow for protein stability analysis in bioprocessing and quality control. This initiative harnesses state-of-the-art analytical instrumentation and software to create a more efficient and reliable environment for assessing protein stability, underscoring Thermo Fisher's dedication to utilizing its expertise in innovative life science technologies. The project highlights the increasing significance of integrated analytical solutions in modern biopharmaceutical manufacturing, contributing to the development of safer, more effective protein drugs
- In February 2023, Charles River Laboratories, a leading contract research organization (CRO), announced an expansion of its biopharmaceutical services, including enhanced capabilities for comprehensive protein stability testing. This strategic move is designed to support growing client demand from biotech and pharmaceutical companies for outsourced stability studies, facilitating more efficient and compliant drug development. The initiative underscores Charles River's commitment to driving innovation and improving operational effectiveness within the biopharmaceutical service sector
- In January 2023, Agilent Technologies, Inc., a leading provider of analytical instrumentation, unveiled new advancements in its chromatography and spectroscopy platforms at major industry conferences. These innovations included features specifically designed to improve the accuracy and throughput of protein stability analysis, enabling users to manage complex protein samples more effectively. The enhancements highlight Agilent's commitment to integrating advanced technology into analytical workflows, offering researchers and developers enhanced precision and control while ensuring robust data for protein characterization
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.