Global Protein Therapeutics Market
Market Size in USD Billion
CAGR :
% 
 USD
131.07 Billion
USD
219.87 Billion
2024
2032
USD
131.07 Billion
USD
219.87 Billion
2024
2032
| 2025 –2032 | |
| USD 131.07 Billion | |
| USD 219.87 Billion | |
|
|
|
|
Protein Therapeutics Market Size
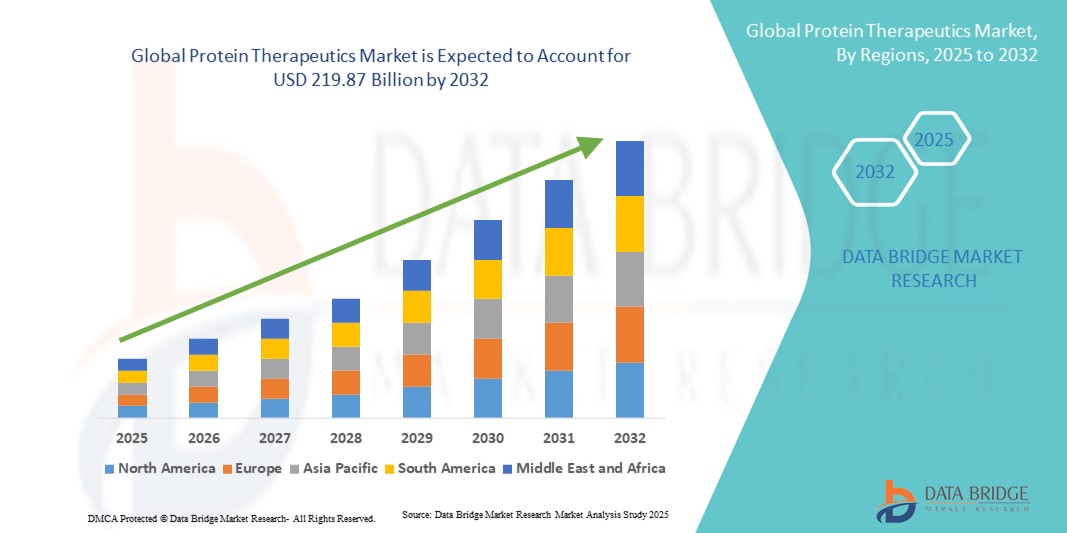
- The global protein therapeutics market size was valued at USD 131.07 billion in 2024 and is expected to reach USD 219.87 billion by 2032, at a CAGR of 6.68% during the forecast period
- The market growth is largely fueled by the rising prevalence of chronic diseases such as cancer, diabetes, and autoimmune disorders, along with the increasing adoption of biologics as effective treatment options. Advancements in biotechnology and protein engineering are enabling the development of highly targeted and personalized therapeutic solutions, driving significant adoption across healthcare systems worldwide
- Furthermore, growing demand for safe, effective, and patient-friendly treatment alternatives is establishing protein therapeutics as a preferred class of biopharmaceuticals. These converging factors, supported by strong R&D investments and expanding approvals of novel biologics, are accelerating the uptake of protein therapeutics solutions, thereby significantly boosting the industry's growth
Protein Therapeutics Market Analysis
- Protein therapeutics, encompassing monoclonal antibodies, insulin analogs, therapeutic enzymes, and other recombinant proteins, are increasingly vital in modern medicine due to their high specificity, reduced side effects, and ability to target complex diseases such as cancer, diabetes, autoimmune disorders, and rare genetic conditions
- The escalating demand for protein therapeutics is primarily fueled by the growing prevalence of chronic diseases, advancements in biotechnology and genetic engineering, and increasing investments in research and development to deliver innovative and patient-centric therapies
- North America dominated the protein therapeutics market with the largest revenue share of 45% in 2024, supported by advanced healthcare infrastructure, high adoption of biologics, strong presence of leading biopharmaceutical companies, and robust regulatory support. The U.S. accounted for the majority of regional revenue, driven by a rising number of FDA approvals, expanded insurance coverage, and increasing patient preference for biologic therapies
- Asia-Pacific is expected to be the fastest-growing region in the protein therapeutics market during the forecast period, owing to rapid urbanization, rising disposable incomes, growing healthcare expenditure, and increasing access to advanced biologic treatments in countries such as China, India, and Japan. Government initiatives to strengthen biotech innovation and favorable clinical research policies are also fueling growth in the region
- The Monoclonal Antibodies segment dominated the protein therapeutics market with the largest revenue share of 42.5% in 2024, owing to their widespread use in treating cancers, autoimmune diseases, and infectious disorders
Report Scope and Protein Therapeutics Market Segmentation
|
Attributes |
Protein Therapeutics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Protein Therapeutics Market Trends
Enhanced Efficacy and Targeted Therapies in Protein Therapeutics
- A significant and accelerating trend in the global protein therapeutics market is the advancement of targeted biologics, particularly monoclonal antibodies, therapeutic enzymes, and recombinant proteins, which provide higher efficacy with fewer side effects compared to traditional drugs. This is reshaping treatment paradigms for cancer, autoimmune diseases, and rare genetic disorders
- For instance, the development of bispecific antibodies and antibody-drug conjugates is revolutionizing oncology by improving tumor targeting and reducing systemic toxicity. Similarly, next-generation insulin analogs are providing better glycemic control and convenience for patients with diabetes
- Continuous R&D investments are enabling the creation of protein therapeutics with improved stability, longer half-life, and enhanced delivery mechanisms. Companies are also exploring fusion proteins and engineered enzymes to expand therapeutic applications in areas such as metabolic and neurological disorders
- The increasing availability of biosimilars is driving affordability and accessibility in the protein therapeutics market, particularly in emerging economies. For instance, several leading pharmaceutical companies are launching biosimilar versions of blockbuster biologics to capture growing demand and reduce treatment costs
- The seamless integration of protein therapeutics into personalized medicine strategies is another emerging trend, with biomarkers and genetic profiling guiding the selection of specific therapies for individual patients. This shift towards precision treatment is improving patient outcomes while expanding market opportunities
- The demand for advanced protein therapeutics is growing rapidly across both developed and developing regions, as healthcare systems increasingly prioritize biologics to manage complex and chronic diseases. Leading companies are responding by expanding clinical pipelines, accelerating regulatory approvals, and investing in large-scale biomanufacturing facilities
Protein Therapeutics Market Dynamics
Driver
Growing Demand Due to Rising Prevalence of Chronic and Rare Diseases
- The global surge in chronic and rare diseases, including cancer, diabetes, autoimmune disorders, and genetic abnormalities, is creating unprecedented demand for advanced therapeutic options. Protein therapeutics, with their ability to target specific molecular pathways, are increasingly being viewed as superior alternatives to conventional small-molecule drug.
- For instance, in March 2024, Amgen reported promising results from its late-stage monoclonal antibody therapy for cardiovascular disease, reinforcing the role of protein-based drugs in addressing large, underserved patient populations
- Unlike traditional drugs, protein therapeutics such as monoclonal antibodies, insulin, interferons, and growth hormones offer greater precision, improved safety profiles, and enhanced efficacy, which is reshaping clinical treatment protocols across specialties
- At the same time, continuous innovation in biotechnology, including recombinant DNA technology and advanced protein engineering, is improving stability, half-life, and drug delivery methods. These innovations are making therapies more effective and patient-friendly
- Rising healthcare expenditure in both developed and emerging economies, coupled with supportive regulatory frameworks encouraging biologic development and approvals, further accelerates the adoption of protein therapeutics. As a result, these therapies are becoming a cornerstone of modern medicine, driving consistent market growth
Restraint/Challenge
High Production Costs and Stringent Regulatory Requirements
- Despite their clinical success, protein therapeutics face significant cost and regulatory barriers that restrict widespread adoption. Manufacturing processes for these biologics are inherently complex, involving advanced cell culture techniques, purification processes, and strict quality control standards. These complexities lead to elevated production costs, making therapies expensive for both healthcare systems and patients
- Moreover, the regulatory landscape surrounding protein-based therapies is highly stringent. Agencies such as the FDA (U.S.) and EMA (Europe) impose rigorous requirements for demonstrating safety, efficacy, and comparability, especially for biosimilars. This often results in prolonged development timelines, high R&D expenditures, and delayed market entry
- An additional challenge lies in the specialized logistics required for protein therapeutics. Many biologics are sensitive to temperature fluctuations, necessitating cold-chain storage and transport, which adds to the overall distribution cost and limits accessibility in resource-constrained regions
- High-profile cases of regulatory delays and quality compliance issues further highlight the barriers companies face in maintaining consistent production standards. These factors collectively contribute to limited affordability and accessibility in developing nations
- Addressing these challenges requires industry-wide efforts to advance cost-efficient bioprocessing technologies, foster biosimilar innovation to reduce costs, and harmonize regulatory frameworks to streamline approvals. Without such measures, the full potential of protein therapeutics may remain constrained despite their strong clinical value
Protein Therapeutics Market Scope
The Protein Therapeutics market is segmented on the basis of Product, Application, End User, and Function
- By Product
On the basis of product, the protein therapeutics market is segmented into monoclonal antibodies, insulin, fusion protein, erythropoietin, interferon, human growth hormone, and follicle stimulating hormone. The monoclonal antibodies segment dominated the market with the largest revenue share of 42.5% in 2024, owing to their widespread use in treating cancers, autoimmune diseases, and infectious disorders. These therapies are highly specific, targeting disease-causing cells while minimizing harm to healthy tissue, which makes them central to precision medicine. Their established role in immunotherapy, continuous regulatory approvals, and expanding pipeline reinforce their dominance. Moreover, the surge in personalized medicine and increasing adoption in both developed and emerging economies have further accelerated demand for monoclonal antibodies.
The Insulin segment is anticipated to witness the fastest CAGR of 9.8% from 2025 to 2032, driven by the global diabetes epidemic affecting more than 500 million people. Insulin is a life-sustaining therapy, and advancements in long-acting formulations and smart insulin delivery devices are enhancing patient compliance. The growing availability of biosimilar insulin is improving affordability, particularly in emerging markets, expanding its accessibility. Continuous innovation in insulin analogs and delivery mechanisms ensures a robust outlook for this segment over the forecast period.
- By Application
On the basis of application, the protein therapeutics market is segmented into metabolic disorders, immunologic disorders, hematological disorders, cancer, hormonal disorders, genetic disorders, and others. The cancer segment accounted for the largest revenue share of 38.9% in 2024, supported by the widespread use of monoclonal antibodies, checkpoint inhibitors, and fusion proteins in oncology. Protein-based therapies are preferred because of their ability to specifically target cancer cells and reduce systemic toxicity compared to conventional chemotherapy. High global incidence of cancer, extensive R&D investments, and growing adoption of personalized treatment regimens have reinforced the dominance of this segment. In addition, continuous clinical trial approvals and new product launches are expected to maintain its leadership.
The immunologic disorders segment is projected to record the fastest CAGR of 10.4% from 2025 to 2032, fueled by the rising prevalence of autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, and psoriasis. Protein therapeutics, especially monoclonal antibodies and fusion proteins, are increasingly being adopted as first-line treatments due to their long-term efficacy and safety profile. Expanding therapeutic indications, coupled with the strong pipeline of biologics targeting immune pathways, are further propelling growth. Patients’ preference for targeted therapies with fewer side effects compared to conventional drugs also strengthens this segment’s trajectory.
- By End User
On the basis of end user, the protein therapeutics market is segmented into pharmaceutical companies, healthcare service providers, research organizations, and academic research institutes. The pharmaceutical companies segment dominated the market with the largest revenue share of 47.2% in 2024, due to their leading role in drug discovery, manufacturing, and commercialization of protein-based therapeutics. These companies invest heavily in clinical trials, regulatory approvals, and pipeline development, ensuring a continuous flow of new therapies to the market. Their extensive global distribution channels, strategic partnerships, and ability to scale production give them a decisive advantage. Moreover, patent exclusivity and blockbuster biologics continue to sustain high revenues for this segment.
The healthcare service providers segment is expected to grow at the fastest CAGR of 8.7% from 2025 to 2032, as hospitals, specialty clinics, and healthcare centers expand their use of protein therapeutics in treatment protocols. Rising patient demand for biologics, coupled with advanced diagnostic tools that enable personalized therapies, has increased adoption. Greater access to biologics in both advanced and emerging healthcare systems, along with improved reimbursement structures, is boosting this segment’s rapid growth during the forecast period.
- By Function
On the basis of function, the protein therapeutics market is segmented into vaccines, enzymatic and regulatory, and protein diagnostics. The vaccines segment dominated the market with the largest revenue share of 40.6% in 2024, driven by the success of recombinant protein-based vaccines and global immunization initiatives. Protein vaccines are valued for their safety, effectiveness, and ability to generate a strong immune response with minimal side effects. The COVID-19 pandemic highlighted the importance of protein-based vaccine platforms, leading to unprecedented investments and accelerated approvals. Growing demand for preventive healthcare and preparedness against future pandemics further reinforce the leadership of this segment.
The protein diagnostics segment is forecasted to witness the fastest CAGR of 9.5% from 2025 to 2032, as demand for precise and rapid disease detection continues to rise. Protein-based diagnostic tools enable accurate identification of disease biomarkers, particularly in oncology, metabolic conditions, and infectious diseases. The growing adoption of personalized medicine, coupled with advancements in proteomics and biomarker discovery, is significantly boosting this segment. In addition, early detection and monitoring through protein diagnostics improve patient outcomes, further fueling market expansion.
Protein Therapeutics Market Regional Analysis
- North America dominated the protein therapeutics market with the largest revenue share of 45% in 2024, supported by advanced healthcare infrastructure, high adoption of biologics, strong presence of leading biopharmaceutical companies, and robust regulatory support.
- Patients in the region benefit from expanded insurance coverage and broad access to monoclonal antibodies, vaccines, and recombinant proteins for chronic and rare diseases
- This widespread adoption is further reinforced by the increasing number of FDA approvals, ongoing clinical trials, and strategic collaborations between pharmaceutical companies and academic institutions. These factors are establishing protein therapeutics as a cornerstone of treatment in oncology, metabolic disorders, and autoimmune diseases
U.S. Protein Therapeutics Market Insight
The U.S. protein therapeutics market captured the majority share in 2024 within North America, fueled by rising demand for biologics and the country’s strong R&D ecosystem. Growth is driven by increasing patient preference for biologic therapies over conventional small-molecule drugs, rapid regulatory approvals, and government initiatives to accelerate innovation in biopharmaceuticals. The U.S. market also benefits from strong reimbursement policies and a high rate of adoption in specialty hospitals and clinics, making it the leading market for protein therapeutics globally.
Europe Protein Therapeutics Market Insight
The Europe protein therapeutics market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by stringent healthcare regulations, rising prevalence of chronic diseases, and increasing demand for advanced biologics. European countries are witnessing strong adoption of monoclonal antibodies, vaccines, and therapeutic enzymes, with applications expanding across oncology, neurology, and metabolic disorders. The region is also experiencing growth in biosimilars, supported by favorable regulatory frameworks from the European Medicines Agency (EMA), which is accelerating patient access to cost-effective biologics.
U.K. Protein Therapeutics Market Insight
The U.K. protein therapeutics market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by rising demand for biologics in oncology and autoimmune diseases. A robust biopharmaceutical research base, government support for biotech innovation, and strong clinical trial infrastructure are supporting the market’s expansion. Furthermore, partnerships between the NHS and pharmaceutical companies are enhancing accessibility and affordability of protein-based therapies, further driving adoption.
Germany Protein Therapeutics Market Insight
The Germany protein therapeutics market is expected to expand at a considerable CAGR during the forecast period, fueled by the country’s emphasis on biotechnology and innovation. Germany’s well-developed healthcare infrastructure, combined with its position as a leading hub for clinical research and biologics manufacturing, supports adoption in oncology, metabolic, and rare disease segments. Increasing investment in biosimilar development and a strong focus on sustainable, cost-effective therapies also align with Germany’s market growth trajectory.
Asia-Pacific Protein Therapeutics Market Insight
The Asia-Pacific protein therapeutics market is poised to grow at the fastest CAGR from 2025 to 2032, driven by rapid urbanization, rising disposable incomes, growing healthcare expenditure, and increasing access to biologic treatments in China, India, and Japan. Government initiatives to strengthen biotech research and favorable clinical trial regulations are accelerating innovation across the region.As APAC emerges as a key hub for biologics manufacturing, the affordability and accessibility of protein therapeutics are expanding, making advanced treatments available to a larger patient base.
Japan Protein Therapeutics Market Insight
The Japan Protein Therapeutics market is gaining momentum due to the country’s advanced healthcare system, strong biotechnology infrastructure, and increasing aging population. The demand for biologics, particularly monoclonal antibodies and recombinant proteins, is rising as part of Japan’s strategy to address chronic diseases and age-related conditions. Integration of protein-based therapies into public healthcare programs and the expansion of domestic biotech companies are fueling growth.
China Protein Therapeutics Market Insight
The China Protein Therapeutics market accounted for the largest share in Asia-Pacific in 2024, attributed to the country’s expanding middle class, rising healthcare spending, and strong government focus on biotechnology. China is one of the largest markets for biologics, with rapid adoption of monoclonal antibodies, vaccines, and biosimilars. The government’s “Healthy China 2030” initiative and favorable policies for biopharmaceutical innovation are supporting strong domestic production and international partnerships, positioning China as a global growth engine for protein therapeutics.
Protein Therapeutics Market Share
The protein therapeutics industry is primarily led by well-established companies, including:
- Abbott (U.S.)
- Amgen Inc. (U.S.)
- Baxter (U.S.)
- Lilly USA, LLC (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Johnson & Johnson and its affiliates (U.S.)
- Merck KGaA (Germany)
- Novo Nordisk A/S (Denmark)
- Pfizer Inc. (U.S.)
- Sanofi (France)
- AstraZeneca (U.K.)
- Boehringer Ingelheim International GmbH (Germany)
- Teva Pharmaceutical Industries Ltd (Israel)
- Kyowa Kirin Co., Ltd. (Japan)
- AbbVie Inc. (U.S.)
- Generex Biotechnology Corp. (U.S.)
- CSL (Australia)
- Biogen Inc. (U.S.)
- Genentech, Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
Latest Developments in Global Protein Therapeutics Market
- In November 2022, the U.S. FDA approved TZIELD (teplizumab-mzwv), marking the first therapy capable of delaying the onset of stage 3 type 1 diabetes. This milestone approval underscored the growing importance of targeted biologic therapies in preventing disease progression
- In June 2023, UCB received FDA approval for RYSTIGGO (rozanolixizumab-noli) for the treatment of generalized myasthenia gravis. This represented a key advance in neuromuscular disease management. The therapy later gained approval in the European Union in January 2024, reflecting its rapid global regulatory acceptance
- In July 2023, the FDA granted traditional approval to LEQEMBI (lecanemab-irmb) for the treatment of early Alzheimer’s disease. This followed its accelerated approval in January 2023, making LEQEMBI one of the most significant biologic advances in addressing neurodegenerative disorders
- In September 2023, the FDA expanded the approval of ABRYSVO (RSV vaccine) for use in maternal immunization, enabling the protection of infants against respiratory syncytial virus. This development reinforced the critical role of protein-based vaccines in safeguarding vulnerable populations
- In March 2024, the FDA updated the labeling for WEGOVY (semaglutide) to include a cardiovascular risk-reduction indication. This marked the first time an anti-obesity therapy carried such a label, significantly broadening its clinical value and reinforcing the therapeutic potential of protein-based treatments in chronic disease management
- In July 2024, the FDA approved KISUNLA (donanemab-azbt), Eli Lilly’s anti-amyloid monoclonal antibody, for the treatment of early Alzheimer’s disease. This approval further strengthened the therapeutic arsenal available for tackling one of the most challenging neurological disorders
- In October 2024, the FDA approved HYMPAVZI (marstacimab), Pfizer’s once-weekly subcutaneous therapy for reducing bleeds in patients with hemophilia A or B. The therapy was also approved in the European Union in November 2024, marking an important step forward in improving convenience and quality of life for hemophilia patients
- In April 2025, the FDA approved IMAAVY (nipocalimab-csqy), developed by Johnson & Johnson, for the treatment of generalized myasthenia gravis. As an FcRn-blocking monoclonal antibody, it introduced a novel mechanism of action to address an autoimmune neuromuscular condition
- In June 2025, the FDA approved ENFLONSIA (clesrovimab), Merck’s long-acting monoclonal antibody for the prevention of RSV in infants. The same month, the U.S. CDC’s Advisory Committee on Immunization Practices (ACIP) issued a recommendation for its use, highlighting its public health significance in protecting newborns and young children
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.