Global Prurigo Nodularis Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
1.26 Billion
USD
1.71 Billion
2024
2032
USD
1.26 Billion
USD
1.71 Billion
2024
2032
| 2025 –2032 | |
| USD 1.26 Billion | |
| USD 1.71 Billion | |
|
|
|
|
Prurigo Nodularis Treatment Market Size
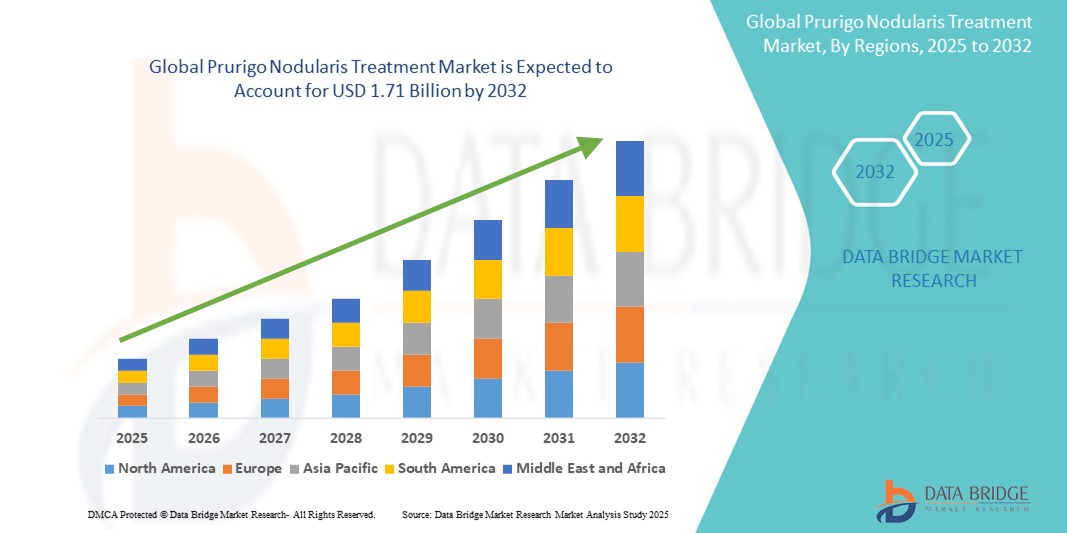
- The global prurigo nodularis treatment market size was valued at USD 1.26 billion in 2024 and is expected to reach USD 1.71 billion by 2032, at a CAGR of 3.86% during the forecast period
- The market growth is largely driven by increasing awareness and diagnosis rates of chronic inflammatory skin disorders, along with the rising prevalence of prurigo nodularis among the aging population and immunocompromised individuals
- Furthermore, the introduction of novel biologic therapies and the growing focus on targeted immunomodulators are establishing advanced treatment options as the preferred clinical approach. These converging factors are accelerating clinical innovation and market penetration, thereby significantly boosting the industry’s growth
Prurigo Nodularis Treatment Market Analysis
- Prurigo nodularis treatments, targeting chronic inflammatory skin conditions marked by intensely itchy nodules, are becoming increasingly vital in dermatological care due to the condition’s significant impact on patient quality of life and the need for effective long-term management strategies
- The growing demand for prurigo nodularis treatments is primarily driven by rising disease awareness, increasing diagnosis rates, and advancements in immunodermatology, particularly the development of biologics and targeted therapies
- North America dominated the prurigo nodularis treatment market with the largest revenue share of 42.2% in 2024, characterized by a high burden of chronic skin conditions, greater access to specialty care, and accelerated FDA approvals of novel therapies such as IL-31 receptor antagonists, with the U.S. witnessing robust adoption across dermatology clinics and specialty centers
- Asia-Pacific is expected to be the fastest growing region in the prurigo nodularis treatment market during the forecast period due to expanding healthcare infrastructure, rising dermatological consultations, and increasing availability of advanced therapies
- Medication segment dominated the prurigo nodularis treatment market with a market share of 58.9% in 2024, driven by its effectiveness in managing symptoms and the growing availability of advanced therapies such as biologics and immunosuppressants
Report Scope and Prurigo Nodularis Treatment Market Segmentation
|
Attributes |
Prurigo Nodularis Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Prurigo Nodularis Treatment Market Trends
“Rising Adoption of Targeted Biologic Therapies”
- A significant and rapidly advancing trend in the global prurigo nodularis treatment market is the growing adoption of targeted biologic therapies, especially IL-31 receptor antagonists and other immunomodulators designed to address the underlying causes of chronic pruritus and inflammation
- For instance, Dupixent (dupilumab), which targets IL-4 and IL-13 pathways, has shown promising clinical outcomes in reducing itch and nodule count in prurigo nodularis patients, leading to its regulatory approvals in multiple regions
- The success of biologics is driving increased clinical research and investment into similar pathway-specific therapies, offering new hope for patients with refractory or severe forms of the disease. In addition, these advanced treatments are raising awareness among healthcare providers about improved standards of care beyond traditional corticosteroids or antihistamines
- Biologic treatments are typically delivered via injection and are being incorporated into long-term disease management plans due to their sustained efficacy and improved patient-reported outcomes. This trend aligns with the shift toward personalized medicine and precision dermatology, where treatments are tailored to specific immune profiles
- The emphasis on targeted, disease-modifying therapies is transforming the treatment landscape and elevating expectations for outcomes in chronic dermatological care. As a result, pharmaceutical companies such as Regeneron and Sanofi are leading developments in this area, while other players are entering clinical trials to expand the portfolio of biologics and immunotherapeutics
- The increasing acceptance of biologic therapies among dermatologists and patients, coupled with growing insurance coverage and favorable reimbursement scenarios, is accelerating the market uptake of these advanced treatment modalities across North America, Europe, and emerging Asia-Pacific regions
Prurigo Nodularis Treatment Market Dynamics
Driver
“Growing Patient Awareness and Advancements in Dermatological Research”
- The rising global awareness of prurigo nodularis as a distinct, debilitating dermatological condition and increasing efforts toward early diagnosis are driving the demand for effective treatment options
- For instance, in 2024, the American Academy of Dermatology launched an educational campaign to improve recognition of chronic pruritic disorders and promote referral pathways for timely intervention, further fueling patient outreach and market expansion
- The availability of novel, evidence-based therapies including biologics and non-steroidal immunomodulators is enhancing patient outcomes and reducing long-term reliance on corticosteroids, which often come with adverse effects
- Simultaneously, the dermatology sector is witnessing robust innovation pipelines, backed by clinical trials focused on cytokine-targeted interventions and nerve-sensitization modulators, expanding the therapeutic arsenal
- This growing therapeutic sophistication is fostering improved disease management and reshaping treatment paradigms, particularly in specialty clinics and hospitals equipped with advanced dermatological expertise
Restraint/Challenge
“High Cost of Advanced Therapies and Limited Access in Emerging Markets”
- Despite strong clinical efficacy, the high cost associated with biologics and other advanced therapies remains a significant barrier, particularly in low- and middle-income regions where access to specialty care is limited
- For instance, biologic therapies such as dupilumab can cost thousands of dollars annually, posing affordability challenges for uninsured patients or healthcare systems with constrained budgets
- Moreover, disparities in dermatological infrastructure and a lack of widespread clinical training on prurigo nodularis further hinder diagnosis and effective treatment, delaying patient access to optimized care
- In addition to economic barriers, regulatory hurdles and limited availability of approved biologics in certain regions prolong patient reliance on less effective conventional therapies, contributing to disease progression
- Addressing these challenges will require broader health policy initiatives, pricing reforms, and global partnerships to enhance affordability and ensure equitable access to advanced treatments for prurigo nodularis worldwide
Prurigo Nodularis Treatment Market Scope
The market is segmented on the basis of treatment, diagnosis, dosage, route of administration, end user and distribution channel.
- By Treatment
On the basis of treatment, the prurigo nodularis treatment market is segmented into medication, cryotherapy, phototherapy, pulsed dye laser, and others. The medication segment dominated the market with the largest market revenue share of 58.9% in 2024, driven by its effectiveness in managing inflammation, itch, and immune dysregulation, as well as the increasing adoption of advanced therapies such as corticosteroids, antihistamines, and biologics. Medications remain the frontline approach for both acute flare-ups and long-term symptom control across various healthcare settings.
The phototherapy segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by increasing acceptance of narrowband UVB and excimer laser therapies in patients with moderate-to-severe disease unresponsive to topicals. Phototherapy offers a steroid-sparing option and is gaining traction among dermatologists for its non-invasive and targeted approach
- By Diagnosis
On the basis of diagnosis, the prurigo nodularis treatment market is segmented into skin biopsy, blood tests, and others. The skin biopsy segment held the largest market revenue share of 46.7% in 2024, driven by its critical role in confirming prurigo nodularis diagnosis, differentiating it from similar dermatologic conditions, and guiding treatment decisions. Skin biopsy remains a routine diagnostic tool in specialized dermatology clinics and tertiary care settings.
The blood tests segment is expected to witness the fastest CAGR from 2025 to 2032, as immunologic and allergy profiles are increasingly evaluated to identify comorbidities and optimize systemic treatment selection, especially for biologics.
- By Dosage
On the basis of dosage, the prurigo nodularis treatment market is segmented into injection, tablets, ointments, and others. The ointment segment dominated the prurigo nodularis treatment market with a market share of 38.9% in 2024, driven by its widespread use as a non-invasive, first-line therapy for localized symptom relief. Topical corticosteroids, calcineurin inhibitors, and emollients are commonly prescribed and preferred by both physicians and patients for initial treatment and flare management.
The injection segment is expected to register the fastest growth rate from 2025 to 2032 due to the rising adoption of biologics administered via subcutaneous injection, offering targeted immune suppression and long-term disease control for severe cases.
- By Route of Administration
On the basis of route of administration, the prurigo nodularis treatment market is segmented into oral, parenteral, topical, and others. The topical segment led the market with the largest market revenue share of 40.2% in 2024, due to its ease of application, lower systemic side effects, and its suitability for early-stage and mild-to-moderate prurigo nodularis. Topical agents form the foundation of therapy and are widely accessible through outpatient care.
The parenteral segment is anticipated to grow at the highest rate during forecast period, propelled by the emergence of targeted biologics such as IL-31 receptor antagonists and their increasing use in treatment-resistant cases requiring systemic immune modulation.
- By End-Users
On the basis of end-users, the prurigo nodularis treatment market is segmented into clinic, hospital, and others. The hospital segment accounted for the largest market revenue share of 44.5% in 2024, driven by access to multidisciplinary dermatology care, biologic therapies, phototherapy units, and diagnostic infrastructure including biopsy and lab testing. Hospitals are also key hubs for clinical trials and the administration of advanced treatments.
The clinic segment is expected to register strong growth over the forecast period, supported by the rise in dermatology-specific outpatient practices and the increasing demand for specialized care with shorter wait times and lower costs compared to hospital settings.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The hospital pharmacy segment dominated the market with the largest market share of 41.8% in 2024, driven by its role in dispensing prescription-only medications such as biologics, immunosuppressants, and high-potency topical agents under direct physician supervision.
The online pharmacy segment is anticipated to grow at the fastest CAGR from 2025 to 2032, supported by rising digital health adoption, convenience in home delivery, and increased access to chronic condition refills and topical therapies, especially in urban regions.
Prurigo Nodularis Treatment Market Regional Analysis
- North America dominated the prurigo nodularis treatment market with the largest revenue share of 42.2% in 2024, characterized by a high burden of chronic skin conditions, greater access to specialty care, and accelerated FDA approvals of novel therapies such as IL-31 receptor antagonists, with the U.S. witnessing robust adoption across dermatology clinics and specialty centers
- Patients and healthcare providers in the region prioritize targeted and long-term management strategies, with high adoption of innovative therapies such as IL-31 receptor antagonists and specialty dermatology consultations
- This regional leadership is further supported by robust healthcare infrastructure, a high concentration of dermatology specialists, favorable reimbursement scenarios, and continuous clinical research, positioning North America as a key hub for both treatment access and innovation in prurigo nodularis care
U.S. Prurigo Nodularis Treatment Market Insight
The U.S. prurigo nodularis treatment market captured the largest revenue share of 82% in 2024 within North America, fueled by high awareness of chronic skin conditions, rapid adoption of biologic therapies, and strong healthcare infrastructure. Patients are increasingly seeking specialized care and targeted treatments such as IL-31 inhibitors and non-steroidal immunomodulators. The presence of major pharmaceutical players, favorable reimbursement policies, and ongoing clinical trials further bolster market growth, positioning the U.S. as a leading hub for innovation and access in prurigo nodularis care.
Europe Prurigo Nodularis Treatment Market Insight
The Europe prurigo nodularis treatment market is projected to expand at a substantial CAGR throughout the forecast period, driven by increasing diagnosis rates, the availability of advanced treatment options, and improved dermatology services across the region. The rise in autoimmune and inflammatory skin disorders, combined with regulatory support for novel therapies, is fostering demand. Countries such as Germany, France, and Italy are seeing growing adoption of biologics and phototherapy, especially in hospital-based dermatology centers and specialty clinics.
U.K. Prurigo Nodularis Treatment Market Insight
The U.K. prurigo nodularis treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by expanding access to dermatological care, NHS-backed treatment pathways, and increasing awareness of pruritic skin diseases. The shift towards biologic and non-steroidal therapies is being driven by both clinicians and patients seeking long-term relief with fewer side effects. Moreover, the presence of research institutes and strong clinical trial participation is contributing to faster adoption of next-generation therapies.
Germany Prurigo Nodularis Treatment Market Insight
The Germany prurigo nodularis treatment market is expected to expand at a considerable CAGR during the forecast period, fueled by the country’s advanced healthcare infrastructure, focus on specialty dermatology, and rising cases of chronic inflammatory skin conditions. Germany’s emphasis on personalized care and innovation is supporting the integration of targeted therapies such as IL-31 receptor antagonists, with hospitals and research centers playing a pivotal role in treatment advancements and patient access to biologics.
Asia-Pacific Prurigo Nodularis Treatment Market Insight
The Asia-Pacific prurigo nodularis treatment market is poised to grow at the fastest CAGR of 23.5% during the forecast period of 2025 to 2032, driven by increased disease awareness, improved access to dermatological care, and rising healthcare investments in countries such as China, Japan, and India. Government-led healthcare reforms and expanding clinical research in chronic skin disorders are accelerating the availability of new therapies. In addition, international pharmaceutical companies are entering the APAC region to tap into its large patient population and unmet treatment needs.
Japan Prurigo Nodularis Treatment Market Insight
The Japan prurigo nodularis treatment market is gaining momentum due to its aging population, high demand for advanced dermatological care, and strong acceptance of innovative therapies. Japan’s healthcare system encourages the adoption of biologics and supports precision medicine approaches. The country's advanced diagnostic capabilities and integration of skin treatment technologies with electronic health records are helping physicians provide more personalized and effective care for prurigo nodularis patients.
India Prurigo Nodularis Treatment Market Insight
The India prurigo nodularis treatment market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to its growing population, expanding middle class, and rapid improvements in dermatological care access. Rising awareness of chronic skin disorders, along with the availability of cost-effective generics and topical formulations, is driving market expansion. India’s increasing participation in clinical trials and partnerships with global pharma companies are expected to further enhance the availability and affordability of advanced therapies.
Prurigo Nodularis Treatment Market Share
The prurigo nodularis treatment industry is primarily led by well-established companies, including:
- Novartis AG (Switzerland)
- AstraZeneca (U.K.)
- Merck & Co., Inc. (U.S.)
- Sumitomo Corporation (Japan)
- AbbVie Inc. (Ireland)
- LEO Pharma A/S (Denmark)
- Cipla (U.S.)
- Lilly (U.S.)
- Bayer AG (Germany)
- Abbott (U.S.)
- Pfizer Inc. (U.S.)
- GSK plc (U.K.)
- Johnson & Johnson Services, Inc. (U.S.)
- Teva Pharmaceutical Industries Ltd. (Jerusalem)
- Sun Pharmaceutical Industries Ltd. (India)
- Aurobindo Pharma (India)
- Lupin (India)
- Bausch Health Companies Inc. (Canada)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Viatris Inc. (U.S.)
What are the Recent Developments in Global Prurigo Nodularis Treatment Market?
- In May 2024, Regeneron Pharmaceuticals Inc. and Sanofi S.A. announced that the U.S. FDA approved Dupixent (dupilumab) for the treatment of prurigo nodularis in adults, making it the first and only biologic approved for this condition. This development marked a major milestone in the management of chronic inflammatory skin diseases, offering patients a targeted therapy that significantly reduces itch and nodule formation, thereby improving quality of life and setting a new standard in dermatologic care
- In April 2024, Galderma reported positive Phase III trial results for its investigational monoclonal antibody lebrikizumab, showing significant improvement in itch relief and nodule regression in prurigo nodularis patients. These results support the growing trend of targeting type 2 inflammation in chronic skin diseases and reinforce Galderma’s commitment to expanding biologic options in dermatology
- In February 2024, VYNE Therapeutics Inc. announced the initiation of a clinical trial to evaluate VYN201, a topical pan-bromodomain inhibitor, for the treatment of prurigo nodularis. This next-generation topical therapy aims to modulate gene expression involved in inflammation and skin thickening. The trial underscores the industry's focus on non-steroidal, localized treatments that offer improved safety profiles and efficacy for patients with refractory disease
- In January 2024, Incyte Corporation disclosed the start of a global Phase II study of ruxolitinib cream, a topical JAK inhibitor, in patients with prurigo nodularis. Given the success of JAK inhibitors in other dermatologic indications, this move reflects the expanding research into precision therapies for chronic pruritic conditions, with potential for both monotherapy and combination use
- In December 2023, the European Academy of Dermatology and Venereology (EADV) released updated clinical guidelines recognizing prurigo nodularis as a distinct and severely burdensome dermatological disease. The guidelines emphasize early diagnosis, stepwise treatment approaches, and inclusion of biologic and immunomodulatory therapies. This development highlights increasing clinical awareness and support for standardized, evidence-based treatment frameworks across Europe
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.