Global Pseudorheumatoid Dysplasia Market
Market Size in USD Billion
CAGR :
% 
 USD
3.12 Billion
USD
4.60 Billion
2024
2032
USD
3.12 Billion
USD
4.60 Billion
2024
2032
| 2025 –2032 | |
| USD 3.12 Billion | |
| USD 4.60 Billion | |
|
|
|
|
Pseudorheumatoid Dysplasia Market Size
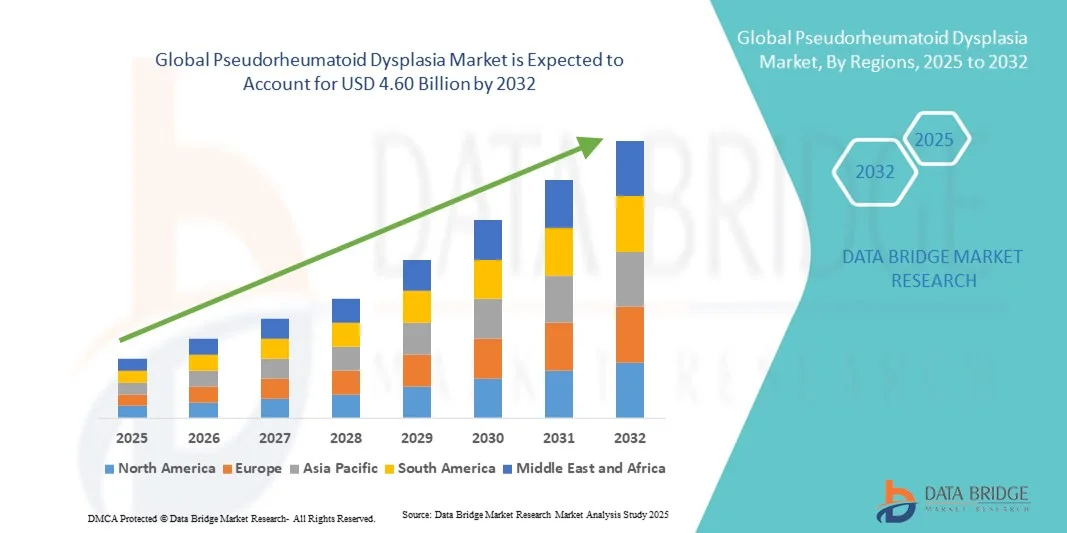
- The global pseudorheumatoid dysplasia market size was valued at USD 3.12 billion in 2024 and is expected to reach USD 4.60 billion by 2032, at a CAGR of 5.00% during the forecast period
- The market growth is primarily driven by the rising prevalence of rare genetic skeletal disorders and the growing advancements in molecular diagnostics, genetic testing, and targeted therapies. Increased research funding and awareness initiatives for early diagnosis are also supporting market expansion
- Furthermore, the growing collaboration between research institutions and biopharmaceutical companies to develop novel therapeutic approaches is accelerating innovation in the field. These combined factors are significantly contributing to the steady growth of the global pseudorheumatoid dysplasia market
Pseudorheumatoid Dysplasia Market Analysis
- Pseudorheumatoid dysplasia, a rare inherited skeletal disorder marked by progressive joint stiffness, gait abnormalities, and short stature, is drawing heightened medical and research focus due to advancements in genetic testing and early diagnosis. Improved awareness among clinicians and patients is enhancing the accuracy of disease detection and patient management worldwide
- The rising demand for effective diagnostic and therapeutic solutions is primarily driven by the expansion of rare disease research initiatives, growing access to advanced molecular diagnostics, and supportive government policies encouraging orphan disease research and treatment development
- North America dominated the pseudorheumatoid dysplasia market with the largest revenue share of 39.8% in 2024, supported by strong research infrastructure, early implementation of genetic screening programs, and the active involvement of leading academic and biopharmaceutical organizations
- Asia-Pacific is expected to be the fastest-growing region in the pseudorheumatoid dysplasia market during the forecast period, fueled by improving healthcare infrastructure, growing awareness of genetic disorders, and expanding collaborations for rare disease diagnostics
- The genetic testing registry (GTR) segment dominated the pseudorheumatoid dysplasia market with a market share of 47% in 2024, driven by the increasing utilization of comprehensive genetic databases and molecular diagnostic tools to confirm disease-causing mutations and enable precise diagnosis
Report Scope and Pseudorheumatoid Dysplasia Market Segmentation
|
Attributes |
Pseudorheumatoid Dysplasia Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Pseudorheumatoid Dysplasia Market Trends
Advancements in Genetic Testing and Early Diagnosis
- A significant and accelerating trend in the global pseudorheumatoid dysplasia market is the growing utilization of advanced genetic testing and molecular diagnostic technologies such as next-generation sequencing (NGS) to enhance early disease detection and clinical decision-making
- For instance, institutions such as the National Institutes of Health (NIH) and Orphanet are increasingly incorporating pseudorheumatoid dysplasia into genetic registries, improving case identification and global data sharing. Similarly, laboratories worldwide are integrating targeted gene panels for WISP3 mutation screening to confirm diagnosis more efficiently
- Genetic testing advancements are enabling clinicians to differentiate pseudorheumatoid dysplasia from other skeletal dysplasias, reducing misdiagnosis rates and improving treatment planning. For instance, collaborations between genetic testing centers and rare disease consortia have enhanced diagnostic precision and patient counseling capabilities
- The integration of diagnostic tools with clinical databases allows healthcare professionals to track patient outcomes and tailor personalized care plans. Through unified platforms, physicians can correlate genotype–phenotype data, facilitating better disease understanding and management strategies
- This trend toward precision diagnostics and genetic data integration is transforming the rare disease landscape, driving the discovery of new therapeutic targets and enhancing awareness. Consequently, companies specializing in rare disease genetics are expanding their offerings to include comprehensive testing and research services
- The rising adoption of advanced molecular diagnostics and registry-based data collection is rapidly shaping the future of pseudorheumatoid dysplasia care, promoting early diagnosis, targeted treatment, and global collaboration among researchers and clinicians
Pseudorheumatoid Dysplasia Market Dynamics
Driver
Growing Research Funding and Orphan Drug Development for Rare Disorders
- The increasing global focus on rare disease research and orphan drug development is a major driver of growth in the pseudorheumatoid dysplasia market, with governments and organizations offering financial incentives and regulatory support
- For instance, in March 2024, the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) expanded funding frameworks for orphan drug development, directly benefiting research on rare skeletal disorders such as pseudorheumatoid dysplasia
- As awareness of genetic skeletal dysplasias grows, patient advocacy networks and research foundations are promoting early diagnosis, genetic counseling, and multidisciplinary treatment approaches to improve quality of life
- Furthermore, advancements in gene sequencing, protein modeling, and bioinformatics are accelerating the discovery of potential therapeutic pathways, attracting investment from biotechnology companies and research institutions
- The increasing collaboration between academia, pharmaceutical firms, and public health agencies is fostering innovation in rare disease treatment, enabling progress toward precision medicine solutions for pseudorheumatoid dysplasia. The growing pipeline of orphan therapies reflects the strengthening ecosystem for rare disorder management
- The integration of supportive policies, R&D funding, and technological progress is expected to further drive innovation and improve access to effective treatment options in the pseudorheumatoid dysplasia market
Restraint/Challenge
Diagnostic Limitations and Low Disease Awareness
- The rarity of pseudorheumatoid dysplasia, coupled with overlapping symptoms with other joint-related disorders, poses significant diagnostic challenges that delay accurate detection and management
- For instance, clinical misidentification as juvenile idiopathic arthritis remains common due to similar joint manifestations, leading to delayed genetic testing and misdirected treatment strategies
- Addressing these diagnostic limitations through clinician education, standardized testing guidelines, and improved access to molecular diagnostics is essential for timely and accurate diagnosis. Companies and organizations such as Orphanet emphasize early genetic screening to minimize diagnostic delays and enhance patient outcomes
- Moreover, the lack of targeted therapies and limited clinical research trials hinder therapeutic advancement, as the small patient pool restricts large-scale studies and commercial interest from major pharmaceutical firms
- The high cost of advanced diagnostic technologies and limited reimbursement coverage in certain regions further restrict accessibility, particularly in developing economies with resource-constrained healthcare systems. While awareness is improving, these challenges still limit early detection and intervention
- Overcoming these barriers through awareness campaigns, policy support, and international collaboration will be vital to improving diagnostic accuracy, research participation, and patient care outcomes in the pseudorheumatoid dysplasia market
Pseudorheumatoid Dysplasia Market Scope
The market is segmented on the basis of symptoms and diagnosis.
- By Symptoms
On the basis of symptoms, the global pseudorheumatoid dysplasia market is segmented into gait disturbance, methylmalonic acidemia, and short stature. The gait disturbance segment dominated the market with the largest revenue share in 2024, primarily due to its high prevalence among patients with pseudorheumatoid dysplasia and its role as one of the earliest recognizable clinical manifestations of the disorder. Gait disturbance often appears during early childhood, leading to orthopedic evaluations and eventual referral for genetic testing. This symptom serves as a critical diagnostic indicator, prompting clinicians to distinguish pseudorheumatoid dysplasia from other juvenile arthropathies. The growing availability of gait analysis tools and motion tracking technologies further enhances clinical understanding and treatment planning. In addition, the emphasis on early mobility rehabilitation and physiotherapy programs is driving demand for accurate assessment and management of gait-related complications.
The short stature segment is anticipated to witness the fastest growth rate from 2025 to 2032, driven by increasing awareness among pediatricians and genetic specialists regarding its correlation with pseudorheumatoid dysplasia and other skeletal dysplasias. Short stature has become a key focus in rare disease screening programs, particularly in specialized pediatric orthopedic and endocrinology centers. The integration of growth monitoring systems with genetic evaluation tools is improving early identification and patient outcomes. Furthermore, advancements in growth pattern analytics and digital health platforms are enabling physicians to monitor developmental anomalies more efficiently. As healthcare systems in emerging markets adopt advanced pediatric screening programs, the demand for precise diagnosis and management of short stature–related rare skeletal disorders is expected to grow substantially.
- By Diagnosis
On the basis of diagnosis, the global pseudorheumatoid dysplasia market is segmented into Genetic Testing Registry (GTR) and Orphanet. The Genetic Testing Registry (GTR) segment dominated the market with the largest revenue share of 47% in 2024, owing to the increasing reliance on comprehensive genetic databases for confirming mutations in the WISP3 gene, which causes pseudorheumatoid dysplasia. GTR offers standardized and accessible genetic information that aids in accurate diagnosis, clinical validation, and global data exchange between laboratories and researchers. The growing adoption of next-generation sequencing (NGS) and whole-exome sequencing (WES) technologies, combined with the expansion of accredited testing facilities, has made GTR a preferred diagnostic resource. In addition, continuous updates and global participation in the registry enhance diagnostic consistency and patient record management, supporting clinical research and epidemiological studies.
The Orphanet segment is expected to witness the fastest growth rate from 2025 to 2032, driven by the platform’s expanding global reach and its growing role in connecting patients, clinicians, and researchers specializing in rare diseases. Orphanet’s integrated approach to collecting, classifying, and disseminating information on rare disorders such as pseudorheumatoid dysplasia facilitates early identification and improved case tracking across regions. The increasing adoption of Orphanet by healthcare providers for rare disease reference and clinical collaboration is further propelling its growth. Moreover, international partnerships and European Union initiatives to strengthen rare disease registries are enhancing Orphanet’s impact in diagnostics, clinical trials, and awareness programs. The platform’s emphasis on patient-centered data and translational research support makes it a rapidly expanding diagnostic avenue in the pseudorheumatoid dysplasia market.
Pseudorheumatoid Dysplasia Market Regional Analysis
- North America dominated the pseudorheumatoid dysplasia market with the largest revenue share of 39.8% in 2024, supported by strong research infrastructure, early implementation of genetic screening programs, and the active involvement of leading academic and biopharmaceutical organizations
- Patients and clinicians in the region benefit from high awareness levels, early diagnostic adoption, and access to leading genetic databases such as GTR and Orphanet, facilitating accurate disease identification and management
- This strong market presence is further supported by significant investment in rare disease research, the availability of specialized treatment centers, and proactive government support for orphan drug development, positioning North America as a key hub for innovation and clinical advancement in pseudorheumatoid dysplasia care
U.S. Pseudorheumatoid Dysplasia Market Insight
The U.S. pseudorheumatoid dysplasia market captured the largest revenue share of 79% in 2024 within North America, fueled by advanced rare disease research infrastructure and early adoption of genetic testing technologies. Increased awareness among healthcare professionals, combined with the presence of key genetic research institutions and patient registries, drives accurate diagnosis and effective management. The expanding availability of next-generation sequencing (NGS) and orphan drug development programs further strengthens market growth. Moreover, supportive FDA frameworks for rare diseases and collaborations between biopharmaceutical companies and research hospitals are significantly contributing to the expansion of this market in the U.S.
Europe Pseudorheumatoid Dysplasia Market Insight
The Europe pseudorheumatoid dysplasia market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by robust government support for rare disease management and the growing number of specialized diagnostic centers. Increasing participation in the European Reference Networks (ERNs) and the adoption of Orphanet for disease data sharing are enhancing diagnostic precision. European healthcare systems are also focusing on improving early detection through national genetic screening programs. Furthermore, collaborative research projects across Germany, France, and the U.K. are accelerating the development of novel diagnostic and therapeutic approaches for pseudorheumatoid dysplasia.
U.K. Pseudorheumatoid Dysplasia Market Insight
The U.K. pseudorheumatoid dysplasia market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by the country’s strong rare disease strategy and increasing public health investment in genetic testing. The National Health Service (NHS) Genomic Medicine Service is playing a crucial role in early identification and patient management. Growing collaboration between universities and biopharmaceutical firms is also fostering research advancements. In addition, increased patient advocacy, combined with active participation in international genetic databases, is helping improve disease awareness and treatment access across the U.K.
Germany Pseudorheumatoid Dysplasia Market Insight
The Germany pseudorheumatoid dysplasia market is expected to expand at a considerable CAGR during the forecast period, fueled by the country’s advanced genetic research ecosystem and strong diagnostic infrastructure. Germany’s commitment to precision medicine and genetic data integration supports early disease detection and classification. The growing presence of rare disease research centers and partnerships with European genetic networks are enhancing clinical outcomes. Moreover, the country’s focus on healthcare innovation and its support for biopharmaceutical R&D are driving the availability of novel diagnostic solutions for pseudorheumatoid dysplasia.
Asia-Pacific Pseudorheumatoid Dysplasia Market Insight
The Asia-Pacific pseudorheumatoid dysplasia market is poised to grow at the fastest CAGR of 22% during the forecast period of 2025 to 2032, driven by increasing awareness of rare diseases, improving healthcare infrastructure, and growing access to genetic testing in countries such as Japan, China, and India. The region’s expanding collaborations with global research institutions and the establishment of rare disease registries are facilitating early diagnosis. Furthermore, government-led digital health initiatives and genetic research funding are enhancing accessibility to diagnostic tools, creating strong growth opportunities in the Asia-Pacific market.
Japan Pseudorheumatoid Dysplasia Market Insight
The Japan pseudorheumatoid dysplasia market is gaining traction due to the country’s advanced medical technology base, strong emphasis on genetic research, and increasing participation in global rare disease studies. Japan’s healthcare policies promoting genomic medicine and early screening are fueling early diagnosis and treatment efforts. The integration of pseudorheumatoid dysplasia into national rare disease databases and patient registries is further improving visibility. Moreover, the growing collaboration between research universities and pharmaceutical companies is accelerating innovation in molecular diagnostics and precision treatment solutions.
India Pseudorheumatoid Dysplasia Market Insight
The India pseudorheumatoid dysplasia market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to the nation’s expanding rare disease awareness programs and strengthening healthcare infrastructure. India’s participation in global genomic initiatives and the establishment of rare disease centers are improving diagnostic access. The rise in genetic counseling services and collaboration with international research networks is fostering early detection and patient support. Moreover, the increasing affordability of molecular diagnostics and government-backed health missions aimed at rare diseases are propelling market growth across the country.
Pseudorheumatoid Dysplasia Market Share
The Pseudorheumatoid Dysplasia industry is primarily led by well-established companies, including:
- Illumina, Inc. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- QIAGEN (Netherlands)
- Invitae Corporation (U.S.)
- Natera, Inc. (U.S.)
- BGI Genomics Co., Ltd. (China)
- Oxford Nanopore Technologies (U.K.)
- Eurofins (Luxembourg)
- Synlab (Germany)
- Stryker (U.S.)
- Zimmer Biomet (U.S.)
- Smith & Nephew (U.K.)
- Johnson & Johnson Services, Inc. (U.S.)
- BioMarin (U.S.)
- Sarepta Therapeutics, Inc. (U.S.)
- Amgen Inc. (U.S.)
- Bionano Genomics, Inc. (U.S.)
- PerkinElmer (U.S.)
- Fulgent Genetics, Inc. (U.S.)
What are the Recent Developments in Global Pseudorheumatoid Dysplasia Market?
- In September 2025, molecular scientists published findings in the International Journal of Molecular Sciences demonstrating that distinct CCN6 gene variants cause variable cellular stress responses, including mitochondrial dysfunction, ER stress, and altered extracellular matrix remodeling in chondrocytes. This research highlights the molecular complexity of PPRD and emphasizes the potential for personalized, mutation-specific therapeutic strategies
- In July 2023, a detailed case study reported a PPRD patient who was initially misdiagnosed with juvenile idiopathic arthritis for several years, later confirmed to have rare compound WISP3 mutations (c.589+2T>C and c.721T>G). The report also documented novel hip and sacroiliac joint involvement not previously described in PPRD, expanding the known clinical phenotype and improving awareness among rheumatologists
- In July 2023, a large-scale Chinese genotype–phenotype study covering 105 patients with progressive pseudorheumatoid dysplasia identified 33 pathogenic CCN6 (WISP3) gene variants, including nine previously unreported mutations. This comprehensive genetic mapping offers valuable insights into population-specific mutation patterns, aiding genetic counseling and diagnostic precision in Asian patients.
- In October 2022, a clinical cohort study conducted in Turkey identified genu varum deformity before age 3 and early loss of ambulation as critical early indicators of PPRD. The study enhances early diagnostic accuracy and helps differentiate PPRD from juvenile idiopathic arthritis, a common source of misdiagnosis, which can significantly improve patient outcomes through earlier intervention
- In September 2022, researchers from Italian institutions published a breakthrough study revealing that mesenchymal stromal cells (MSCs) derived from a PPRD patient show abnormal osteogenic differentiation and disrupted extracellular matrix organization. This discovery identifies a new cellular mechanism underlying the disease and provides a foundation for exploring regenerative or cell-based therapies for pseudorheumatoid dysplasia
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.