Global Recombinant Veterinary Vaccine Market
Market Size in USD Billion
CAGR :
% 
 USD
1.37 Billion
USD
2.39 Billion
2024
2032
USD
1.37 Billion
USD
2.39 Billion
2024
2032
| 2025 –2032 | |
| USD 1.37 Billion | |
| USD 2.39 Billion | |
|
|
|
Recombinant Veterinary Vaccine Market Analysis
The recombinant veterinary vaccine market is experiencing significant growth due to the increasing prevalence of animal diseases and the rising demand for effective preventive measures in both livestock and companion animals. As veterinary healthcare evolves, recombinant vaccines offer advantages such as enhanced safety, targeted immunity, and reduced risks compared to traditional vaccines. The growing awareness of zoonotic diseases and the potential for these diseases to spread to humans also drives the demand for robust vaccination solutions.
Technological advancements in recombinant DNA technology and virus-like particle platforms have paved the way for the development of highly specific and effective vaccines. This is particularly crucial in sectors such as aquaculture and livestock, where diseases such as foot-and-mouth disease and avian influenza can cause devastating economic losses.
The market is further bolstered by increasing investments in veterinary healthcare, alongside stricter regulations regarding animal welfare and biosecurity. Government initiatives and funding are also driving innovation, as they encourage the development of new recombinant vaccines for emerging diseases.
However, challenges remain, such as high production costs and the complexities involved in large-scale manufacturing. The regulatory approval process for recombinant vaccines is lengthy, which can delay market entry. Despite these hurdles, the overall market outlook remains positive, with a growing focus on preventive animal healthcare solutions.
Recombinant Veterinary Vaccine Market Size
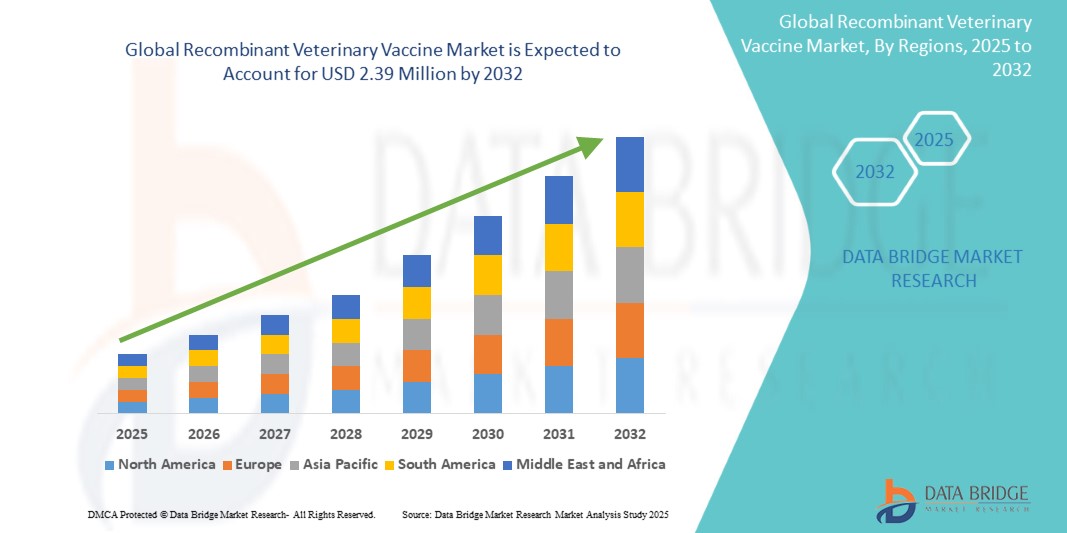
The global recombinant veterinary vaccine market size was valued at USD 1.37 billion in 2024 and is projected to reach USD 2.39 billion by 2032, with a CAGR of 7.22% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Recombinant Veterinary Vaccine Market Trends
“Increasing Adoption of Needle-Free Vaccine Delivery Systems”
One prominent trend in the recombinant veterinary vaccine market is the increasing adoption of needle-free vaccine delivery systems. These systems offer a more efficient, safer, and less stressful method of administering vaccines to animals, particularly in large-scale livestock operations. Needle-free delivery methods, such as jet injectors or oral vaccines, reduce the risk of needle-stick injuries to workers, which is a significant safety concern in veterinary care. They also minimize the need for handling, making vaccination easier and faster, especially in high-density farming environments.
This trend is particularly gaining traction in the poultry and swine industries, where rapid vaccination of large numbers of animals is essential. In addition, needle-free systems help ensure uniform vaccine distribution, which improves efficacy and overall herd immunity. As a result, this technology is expected to drive market growth, as it enhances both the economic viability and accessibility of recombinant veterinary vaccines.
Report Scope and Recombinant Veterinary Vaccine Market Segmentation
|
Attributes |
Recombinant Veterinary Vaccine Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America |
|
Key Market Players |
Bayer AG (Germany), BioNTech SE (Germany), Boehringer Ingelheim International GmbH (Germany), Biogénesis Bagó S.A. (Argentina), Ceva (France), Elanco or its affiliates (U.S.), FeedVax, Inc. (Argentina), HESTER BIOSCIENCES LIMITED (India), Heska Corporation (U.S.), INOVIO Pharmaceuticals (U.S.), Indian Immunologicals Ltd. (India), LABORATORIO AVI-MEX, SA DE CV (Mexico), Merck & Co., Inc. (U.S.), Phibro Animal Health Corporation (U.S.), Virbac (France), VAKSINDO ANIMAL HEALTH PVT. LTD (India), Virbac (France), Vetoquinol (France), Vaccine Valley (Egypt) and Zoetis Services LLC (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Recombinant Veterinary Vaccine Market Definition
A recombinant veterinary vaccine is a type of vaccine developed using recombinant DNA technology, where genes from a pathogen (such as bacteria or viruses) are inserted into a host organism (such as bacteria or yeast) to produce the pathogen's proteins or antigens. These proteins or antigens are then used to stimulate an immune response in animals, helping to protect them from specific diseases. Unlike traditional vaccines, which often use inactivated or attenuated forms of the pathogen, recombinant vaccines offer a safer and more targeted approach by using only the essential components needed for immunity. This technology enables the development of vaccines with improved safety profiles, greater efficacy, and fewer side effects for animals, making it an important advancement in veterinary medicine.
Recombinant Veterinary Vaccine Market Dynamics
Drivers
- Increase in Zoonotic Diseases
The growing incidence of zoonotic diseases, which are transmitted from animals to humans, is a significant driver of the recombinant veterinary vaccine market. Diseases such as avian influenza, rabies, and bovine tuberculosis pose major public health risks, prompting governments and health organizations to focus on controlling their spread through vaccination. Recombinant veterinary vaccines provide a safer and more targeted approach to prevent such diseases. For instance, the recombinant rabies vaccine has been crucial in controlling the disease in wildlife populations, reducing the risk of transmission to humans. As awareness of zoonotic threats increases, the demand for effective veterinary vaccines is expected to rise, boosting market growth. The need for preventive measures against these diseases not only enhances animal health but also helps mitigate the risk of human outbreaks, reinforcing the importance of investing in recombinant veterinary vaccine development.
- Advances in Biotechnology
Advances in biotechnology, especially in recombinant DNA and protein expression technologies, have led to the development of more effective and safe veterinary vaccines. The ability to create vaccines using specific pathogen components rather than whole pathogens improves the safety profile of these vaccines. For instance, recombinant vaccines such as the one for Marek's disease in poultry, which uses a modified virus to stimulate an immune response without causing disease, have demonstrated enhanced efficacy compared to traditional vaccines. As these biotechnological innovations continue to evolve, they enable the creation of vaccines for a wider range of animal diseases, leading to greater market opportunities. The continuous advancement in vaccine technology is likely to drive market growth by offering more specialized solutions for animal health while improving productivity in livestock and aquaculture industries.
Opportunities
- Expansion of Aquaculture Industry
The rapid growth of the global aquaculture industry presents a significant opportunity for the recombinant veterinary vaccine market. As the demand for seafood increases, fish farming has become a major source of protein. However, aquaculture faces numerous challenges, including outbreaks of diseases such as vibriosis and furunculosis, which can lead to significant economic losses. Recombinant vaccines tailored for fish species, such as the recombinant vaccines for vibriosis in salmon, offer a highly effective solution to prevent these diseases. The development and commercialization of such vaccines are expected to increase in response to the expanding aquaculture sector. As fish farming practices scale up to meet global food demands, the adoption of recombinant vaccines will be crucial in improving the health of farmed fish, enhancing production efficiency, and reducing the use of antibiotics. This growing need for effective disease prevention in aquaculture drives market expansion.
- Rising Animal Health Awareness in Emerging Markets
As income levels rise and animal husbandry practices modernize in emerging markets such as Asia-Pacific, Latin America, and Africa, there is a growing awareness of the importance of animal health and preventive measures. This shift is creating new opportunities for recombinant veterinary vaccines. For instance, China and India are increasingly adopting recombinant vaccines for poultry, which help control diseases such as avian influenza and Newcastle disease. This trend is driven by a growing middle class demanding safer, higher-quality animal products. As emerging markets ramp up their veterinary healthcare infrastructure, there is significant potential for recombinant vaccine adoption. This trend is expected to drive the market by encouraging vaccine manufacturers to develop tailored solutions for these regions, ultimately expanding market reach and improving animal health outcomes in these rapidly developing areas.
Restraints/Challenges
- High Production and Development Costs
One of the primary restraints in the recombinant veterinary vaccine market is the high cost of production and development. Developing recombinant vaccines requires advanced biotechnological techniques, which are both time-consuming and expensive. The process involves genetic engineering, rigorous testing, and regulatory approvals, all of which increase the overall cost. For instance, recombinant vaccines for diseases such as Foot-and-Mouth Disease in livestock require significant investment in research and manufacturing capabilities. The high cost of production may limit the availability of these vaccines in price-sensitive markets or small-scale farmers who cannot afford them. This financial barrier could slow the adoption of recombinant vaccines, particularly in developing regions where cost-effective alternatives are more appealing. Consequently, the market growth may be hindered by these high costs, which limit the widespread use of these vaccines in certain geographic regions.
- Regulatory Hurdles
A significant challenge in the recombinant veterinary vaccine market is the lengthy and complex regulatory approval process. Governments and regulatory bodies require extensive safety and efficacy trials before approving new vaccines, especially those produced using recombinant DNA technology. For instance, vaccines such as the recombinant vaccine for rabies require years of testing and validation before they can be introduced to the market. This extended approval timeline can delay the introduction of new vaccines, which poses a challenge to manufacturers aiming to meet the rising demand for animal health solutions. The unpredictability of regulatory pathways can lead to uncertainties in the market, affecting investments and slowing down product commercialization. This challenge could negatively impact market growth, as the ability to quickly deploy new and improved vaccines is crucial for responding to emerging diseases and meeting market demands.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recombinant Veterinary Vaccine Market Scope
The market is segmented on the basis of type of recombinant vaccine, animal type, route of administration, and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type of Recombinant Vaccine
- Subunit Vaccines
- Genetically Attenuated Vaccines
- Vectored Vaccine
- Bacterial vectors
- Viral vectors
- Yeast vectors
- DNA Vaccine
Animal Type
- Dogs
- Cats
- Horses
Route of Administration
- Oral
- Injectable
- Topical
Distribution Channel
- Veterinary Hospitals
- Clinics
Recombinant Veterinary Vaccine Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, type of recombinant vaccine, animal type, route of administration, and distribution channel as referenced above.
The countries covered in the market report are U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America.
North America is expected to dominate the recombinant veterinary vaccine market due to its advanced veterinary healthcare infrastructure, high adoption of innovative technologies, and strong regulatory frameworks. The presence of major players, significant investments in research and development, and a growing focus on animal health contribute to the region’s leadership. In addition, increasing awareness of zoonotic diseases and the demand for preventive animal healthcare further support market growth in North America, driving its dominance in the global market.
Asia-Pacific is expected to exhibit the highest growth rate in the recombinant veterinary vaccine market. This is driven by rapid industrialization in livestock farming, increasing demand for animal protein, and rising awareness about animal health and disease prevention. Countries such as China and India are investing heavily in veterinary healthcare and adopting advanced vaccination technologies. In addition, the growing aquaculture industry in the region further boosts the demand for recombinant veterinary vaccines, fostering rapid market growth.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Recombinant Veterinary Vaccine Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Recombinant Veterinary Vaccine Market Leaders Operating in the Market Are:
- Bayer AG (Germany)
- BioNTech SE (Germany)
- Boehringer Ingelheim International GmbH (Germany)
- Biogénesis Bagó S.A. (Argentina)
- Ceva (France)
- Elanco or its affiliates (U.S.)
- FeedVax, Inc. (Argentina)
- HESTER BIOSCIENCES LIMITED (India)
- Heska Corporation (U.S.)
- INOVIO Pharmaceuticals (U.S.)
- Indian Immunologicals Ltd. (India)
- LABORATORIO AVI-MEX, SA DE CV (Mexico)
- Merck & Co., Inc. (U.S.)
- Phibro Animal Health Corporation (U.S.)
- Virbac (France)
- VAKSINDO ANIMAL HEALTH PVT. LTD (India)
- Virbac (France)
- Vetoquinol (France)
- Vaccine Valley (Egypt)
- Zoetis Services LLC (U.S.)
Latest Developments in Recombinant Veterinary Vaccine Market
- In September 2024, Merck Animal Health announced the expansion of its newly USDA-approved NOBIVAC NXT vaccine platform to include a top-tier solution for protecting cats against feline leukemia virus (FeLV), one of the most prevalent infectious diseases in felines. The vaccine is expected to be available at veterinary clinics and hospitals across the country this fall. NOBIVAC NXT FeLV is the first and only vaccine for feline leukemia virus developed using Merck Animal Health’s RNA-particle technology platform, designed to provide enhanced protection.
- In June 2024, Merck Animal Health announced the launch and availability of the NOBIVAC NXT Rabies portfolio in Canada, which includes NOBIVAC NXT Feline-3 Rabies and NOBIVAC NXT Canine-3 Rabies. This release is part of the company’s ongoing commitment to rabies prevention. The NOBIVAC NXT rabies portfolio is the first-ever line of vaccines that utilizes advanced RNA-particle technology to protect cats and dogs from rabies.
- In February 2024, the University of Pennsylvania School of Veterinary Medicine (Penn Vet) unveiled the launch of its mRNA Research Initiative. This initiative aims to accelerate the development of mRNA-based veterinary vaccines and host-directed therapies. Not only will it make a significant contribution to mRNA research, but it will also help apply the mRNA platform to create innovative veterinary vaccine solutions.
- In July 2023, Invetx announced the successful completion of the development of its species-specific, half-life extension technology for dogs and cats, resulting in a longer duration of activity for the company's veterinary monoclonal antibodies. This innovative technology allows the company to enhance antibody treatment options for chronic and severe diseases in pets. Invetx has been granted four U.S. patents for its half-life extension technology and has additional patents pending in the U.S., Europe, and other regions.
- In September 2021, Invetx announced the launch of a clinical field study in dogs aimed at an undisclosed chronic condition, using IVX-01, its proprietary, novel, fully canine, high-affinity, and half-life extended monoclonal antibody (mAb).
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.