Global Respiratory Syncytial Virus Infection Market
Market Size in USD Billion
CAGR :
% 
 USD
1.92 Billion
USD
4.47 Billion
2024
2032
USD
1.92 Billion
USD
4.47 Billion
2024
2032
| 2025 –2032 | |
| USD 1.92 Billion | |
| USD 4.47 Billion | |
|
|
|
|
Respiratory Syncytial Virus Infection Market Size
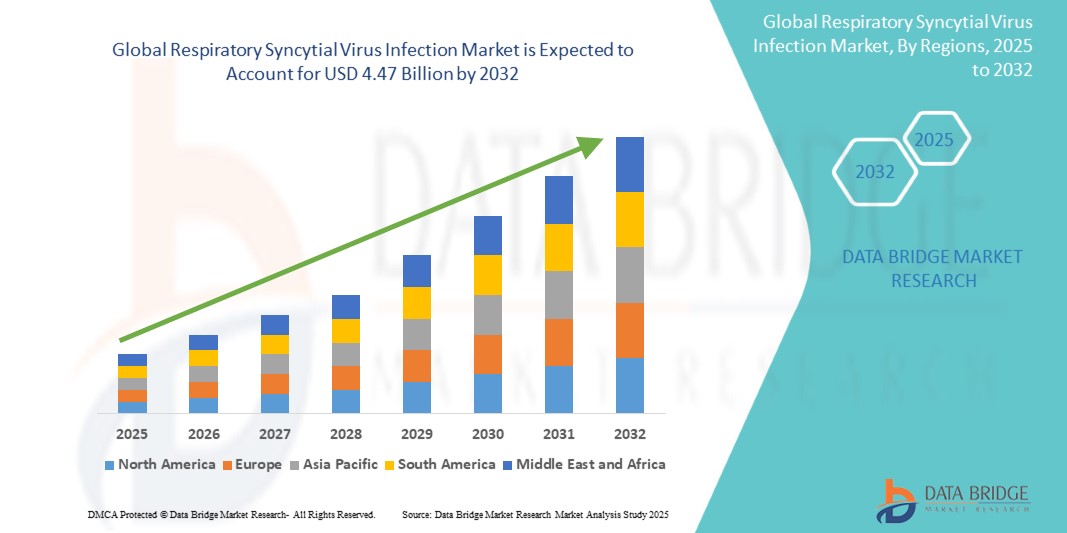
- The global respiratory syncytial virus (RSV) infection market size was valued at USD 1.92 billion in 2024 and is expected to reach USD 4.47 billion by 2032, growing at a CAGR of 11.10% during the forecast period.
- The market growth is largely driven by the increasing prevalence of RSV infections among infants and elderly populations, combined with the rising demand for early diagnosis and effective treatment across healthcare settings.
- Furthermore, growing awareness of RSV-related complications, enhanced access to point-of-care diagnostics, and innovations in antiviral therapies and preventive vaccines are significantly accelerating the adoption of RSV-related healthcare solutions, thereby strengthening market expansion on a global scale.
Respiratory Syncytial Virus Infection Market Analysis
- Respiratory Syncytial Virus (RSV) infection represents a major public health concern, particularly among infants, young children, and elderly adults, due to its potential to cause severe respiratory illness, including bronchiolitis and pneumonia. The virus’s seasonal nature and high hospitalization rates among vulnerable populations are making RSV a critical target for prevention and treatment strategies in the global healthcare landscape.
- The rising demand for effective RSV therapeutics is primarily driven by increasing RSV incidence worldwide, heightened clinical awareness, and the growing availability of advanced diagnostics and care options, such as point-of-care swab tests and pulse oximetry monitoring.
- North America dominates the RSV infection market with the largest revenue share of 37.8% in 2025, supported by a well-established healthcare infrastructure, strong reimbursement environment, and proactive vaccine development efforts. The U.S., in particular, is witnessing significant investment in monoclonal antibody therapies and maternal immunization programs, which is expected to expand further as more RSV vaccines and treatments receive regulatory approvals.
- Asia-Pacific is anticipated to be the fastest-growing region in the RSV infection market during the forecast period, owing to rising birth rates, improved access to diagnostics, and increased healthcare spending in emerging economies such as India and China. Additionally, government-led infant immunization campaigns and awareness programs are contributing to higher RSV diagnosis and treatment uptake.
- Among treatment modalities, supportive care dominates the market with a revenue share of 58.3% in 2025, owing to its foundational role in managing RSV symptoms such as oxygen therapy, hydration, and fever control. However, the demand for advanced antiviral therapies and monoclonal antibodies is increasing rapidly, especially in high-risk patient groups, which will reshape the market landscape in the years ahead.
Report Scope and Respiratory Syncytial Virus Infection Market Segmentation
|
Attributes |
Respiratory Syncytial Virus Infection Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Respiratory Syncytial Virus Infection Market Trends
“Accelerated Advancements in RSV Vaccines and Monoclonal Antibodies”
- A major emerging trend in the global RSV infection market is the surge in development and commercialization of vaccines and long-acting monoclonal antibodies, particularly aimed at vulnerable groups including infants, older adults, and immunocompromised individuals. This trend is reshaping the prevention landscape of RSV, which previously relied heavily on supportive care.
- In 2023, Pfizer received FDA approval for Abrysvo, an RSV vaccine for maternal immunization, and GSK launched Arexvy for older adults, marking a significant leap in preventive healthcare. These approvals have sparked accelerated R&D investments and strategic collaborations across pharmaceutical companies.
- New monoclonal antibodies like nirsevimab (marketed as Beyfortus by AstraZeneca and Sanofi) are designed for broader use across all infants—not just high-risk ones—providing passive immunity with a single dose. These developments are expected to significantly reduce RSV hospitalizations among newborns.
- The trend of RSV diagnostics and vaccines being integrated into broader pediatric and geriatric immunization programs is gaining traction globally, especially as governments and regulatory agencies begin endorsing large-scale RSV surveillance and prevention measures.
- Pharma players are also leveraging platform technologies, such as mRNA and recombinant protein-based vaccines, to speed up development timelines and improve vaccine efficacy. Companies like Moderna and Bavarian Nordic are expected to shape the next wave of RSV vaccines using such technologies.
- This innovation wave in both prophylactic and therapeutic segments is expected to not only transform patient outcomes but also expand the market scope well beyond seasonal infection management.
Respiratory Syncytial Virus Infection Market Dynamics
Driver
“High Disease Burden and Increasing Hospitalizations Among Infants and the Elderly”
- The RSV infection market is primarily driven by the high incidence of lower respiratory tract infections, particularly in children under five and adults above 65. RSV remains one of the leading causes of hospitalization in infants worldwide, accounting for more than 3 million cases annually according to WHO estimates.
- In regions such as North America and Europe, RSV seasonality results in significant healthcare strain each winter, with surges in pediatric ICU admissions and ventilator use. This has created an urgent demand for proactive immunization strategies and effective outpatient treatments.
- Pharmaceutical companies are responding with targeted vaccines and antibody therapies that aim to lower hospitalization rates. For example, the successful Phase III trials of mRNA-based RSV vaccines by Moderna are paving the way for next-gen immunization programs.
- In parallel, the growing inclusion of RSV diagnostics in multiplex respiratory panels (alongside influenza and COVID-19) is driving early detection, better triage, and improved health outcomes.
- These developments, coupled with government-backed vaccine rollouts and rising public awareness, are expected to elevate the global RSV market trajectory in the coming years.
Restraint/Challenge
“High Cost of Biologics and Limited Access in Low-Resource Settings”
- One of the critical restraints of the RSV infection market is the high cost and limited accessibility of advanced biologics, including monoclonal antibodies and novel vaccines, especially in low-income and middle-income countries.
- Products like palivizumab, while effective, are prohibitively expensive for widespread use in non-high-risk populations, restricting their impact. Although newer options like nirsevimab aim to reduce dosing frequency, pricing remains a barrier for national immunization programs.
- In addition, cold chain logistics and lack of healthcare infrastructure in many countries hinder the timely deployment of RSV interventions, particularly in remote or rural regions.
- Another challenge lies in regulatory timelines and approval delays across different regions, which can impede the synchronized global rollout of innovative therapies. The requirement for pediatric and maternal safety data further prolongs the commercialization process.
- Overcoming these challenges will require global partnerships, tiered pricing strategies, and support from international health organizations to ensure equitable access to RSV diagnostics and therapeutics..
Respiratory Syncytial Virus Infection Market Scope
The market is segmented on the basis of diagnosis, treatment, end-users, and distribution channel.
• By Diagnosis
On the basis of diagnosis, the RSV infection market is segmented into blood test, chest X-ray, swab test, pulse oximetry, and others. The swab test segment dominates the largest market revenue share in 2025, owing to its routine use as a frontline diagnostic tool for confirming RSV infection, especially in pediatric and geriatric populations. Swab tests, including rapid antigen and RT-PCR formats, are highly preferred due to their accuracy, ease of sample collection, and fast turnaround time, which are critical for timely treatment.
The pulse oximetry segment is expected to witness the fastest CAGR of 20.5% from 2025 to 2032, driven by its increasing use in monitoring blood oxygen saturation levels in suspected RSV cases. It is especially valuable in emergency care and at-home monitoring setups to detect hypoxemia in infants and elderly patients with compromised respiratory function.
• By Treatment
On the basis of treatment, the RSV infection market is segmented into supportive care, hospital care, and others. The supportive care segment dominates the market share in 2025, accounting for 68.4% of the global market revenue. This is attributed to its foundational role in managing RSV symptoms through oxygen therapy, fluid replacement, nebulization, and fever control. Supportive care remains the primary line of treatment, particularly in the absence of widely accessible antiviral medications.
The hospital care segment is anticipated to witness the fastest CAGR from 2025 to 2032, due to the rising number of severe RSV cases that require inpatient management, especially among neonates, immunocompromised individuals, and older adults. Increasing awareness, coupled with improved access to specialized care units, is fueling the demand for advanced hospital-based interventions.
• By End-Users
On the basis of end-users, the RSV infection market is segmented into hospitals, specialty clinics, and others. The hospital segment held the largest market revenue share in 2025, supported by high patient footfall, availability of ICU facilities, and the presence of multidisciplinary teams for managing moderate to severe RSV infections. Hospitals remain the go-to choice for diagnosing, treating, and monitoring high-risk patients, especially during seasonal RSV outbreaks.
The specialty clinics segment is expected to grow at the fastest CAGR during the forecast period, driven by the increasing role of pediatric, pulmonary, and infectious disease clinics in RSV prevention and early intervention. The expansion of outpatient monoclonal antibody treatment and vaccination programs is also contributing to this growth.
• By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacy, retail pharmacy, online pharmacy, and others. The hospital pharmacy segment dominates the largest market share in 2025, attributed to the direct linkage between inpatient treatments and in-hospital drug dispensing. Most RSV-related therapies, including nebulized bronchodilators and monoclonal antibodies, are administered or initiated in hospitals, thereby making hospital pharmacies the primary point of distribution.
The online pharmacy segment is projected to witness the fastest CAGR from 2025 to 2032, supported by the global shift toward e-commerce, increased telemedicine usage, and growing patient preference for doorstep medication delivery. Online pharmacies are especially gaining traction in urban areas where RSV-related medications and support kits are being purchased for home-based care.
Respiratory Syncytial Virus Infection Market Regional Analysis
- North America dominates the global RSV infection market with the largest revenue share of 38.7% in 2024, driven by high awareness levels, well-established healthcare infrastructure, and strong government support for infectious disease management and immunization programs.
- The region’s robust diagnostic capabilities, presence of leading pharmaceutical companies, and early adoption of RSV prophylaxis and treatment therapies contribute significantly to market leadership.
- Furthermore, the growing burden of RSV-related hospitalizations, especially among infants and older adults, has spurred increased investments in vaccine development and monoclonal antibody therapies, strengthening the regional market position.
U.S. Respiratory Syncytial Virus Infection Market Insight
The U.S. RSV infection market captured the largest revenue share of 80.6% within North America in 2025, driven by a high prevalence of RSV among infants and the elderly, advanced healthcare infrastructure, and increased access to diagnostic and treatment solutions. The widespread use of RSV diagnostics like PCR and antigen tests, along with growing adoption of monoclonal antibody therapies such as nirsevimab and palivizumab, is strengthening market penetration. In addition, the U.S. FDA’s proactive approval of RSV vaccines and therapeutics is enabling faster market growth and innovation.
Europe Respiratory Syncytial Virus Infection Market Insight
The European RSV infection market is projected to grow at a substantial CAGR during the forecast period, fueled by a surge in RSV hospitalizations among pediatric populations and elderly individuals with comorbidities. Enhanced surveillance systems, public health funding, and seasonal immunization campaigns are significantly contributing to market expansion. Countries like Germany, France, and Italy are increasingly adopting RSV prophylactic strategies in neonatal and high-risk adult care settings, driving demand for both diagnostics and biologics.
U.K. Respiratory Syncytial Virus Infection Market Insight
The U.K. RSV infection market is anticipated to grow at a noteworthy CAGR, owing to the National Health Service's (NHS) focus on early RSV detection and timely treatment interventions. Increased public awareness, expansion of RSV vaccine trials, and integration of RSV surveillance into broader respiratory illness tracking systems are boosting growth. The U.K. is also actively participating in clinical research for RSV therapeutics, further accelerating market momentum.
Germany Respiratory Syncytial Virus Infection Market Insight
The German RSV infection market is expected to grow at a considerable CAGR, driven by government investments in pediatric and elderly healthcare, high diagnosis rates, and well-developed reimbursement frameworks. Germany’s focus on preventive care, coupled with strong collaborations between pharmaceutical companies and academic institutions, is supporting the introduction of advanced RSV therapeutics and diagnostics. Hospital-based programs for early RSV detection in neonatal intensive care units are contributing significantly to demand.
Asia-Pacific Respiratory Syncytial Virus Infection Market Insight
The Asia-Pacific RSV infection market is projected to grow at the fastest CAGR of over 23.8% in 2025, propelled by rising birth rates, increased RSV burden, and growing public health awareness in countries like China, India, and Japan. Improving healthcare infrastructure, expanding vaccination coverage, and rising investments from global biopharmaceutical companies are key market drivers. Regional efforts to reduce RSV-related infant mortality are translating into higher demand for monoclonal antibody therapies and diagnostics.
Japan Respiratory Syncytial Virus Infection Market Insight
The Japan RSV infection market is gaining traction due to the country’s aging population, high healthcare spending, and strong surveillance systems for infectious diseases. Widespread use of advanced diagnostic technologies and the rollout of seasonal RSV prevention programs in pediatric clinics are propelling market growth. Japan is also witnessing growing partnerships between domestic pharma players and global firms to accelerate RSV vaccine development.
China Respiratory Syncytial Virus Infection Market Insight
The China RSV infection market accounted for the largest revenue share in Asia-Pacific in 2025, driven by a high pediatric population base, increasing awareness of infant health, and rising healthcare investments. Government-backed immunization programs and rapid expansion of hospital infrastructure are enhancing early detection and intervention for RSV. Domestic pharmaceutical manufacturers are also actively investing in developing cost-effective RSV treatments, further supporting market expansion across both urban and rural regions.
Respiratory Syncytial Virus Infection Market Share
The Respiratory Syncytial Virus Infection industry is primarily led by well-established companies, including:
- AstraZeneca plc (UK)
- Pfizer Inc. (U.S.)
- Sanofi (France)
- GlaxoSmithKline plc (GSK) (UK)
- Moderna, Inc. (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- Bavarian Nordic A/S (Denmark)
- F. Hoffmann-La Roche AG (Switzerland)
- Novavax, Inc. (U.S.)
- AbbVie Inc. (U.S.)
- Sinovac Biotech Ltd. (China)
- BioNTech SE (Germany)
- Bharat Biotech International Limited (India)
- Emergent BioSolutions Inc. (U.S.)
Latest Developments in Global Respiratory Syncytial Virus Infection Market
- In July 2023, Pfizer received FDA approval for Abrysvo, its respiratory syncytial virus (RSV) vaccine. The vaccine is approved for use in older adults and pregnant women, helping protect infants through maternal immunization. This marks a significant advancement in RSV prevention, addressing high-risk groups and reducing severe RSV-related complications in newborns during their early months.
- In November 2022, GSK’s Arexvy became the first RSV vaccine approved by the FDA for adults aged 60 and above. The vaccine aims to reduce the risk of severe lower respiratory tract disease caused by RSV, particularly in older adults who are more vulnerable to serious complications. Arexvy's approval represents a key milestone in adult RSV protection.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.