Global Respiratory Syncytial Virus Vaccines Market
Market Size in USD Billion
CAGR :
% 
 USD
1.47 Billion
USD
2.07 Billion
2024
2032
USD
1.47 Billion
USD
2.07 Billion
2024
2032
| 2025 –2032 | |
| USD 1.47 Billion | |
| USD 2.07 Billion | |
|
|
|
Respiratory Syncytial Virus Vaccines Market Analysis
The respiratory syncytial virus (RSV) vaccine market is experiencing significant growth, driven by advancements in vaccine technology and the increasing recognition of the virus's impact on vulnerable populations, including infants, the elderly, and individuals with compromised immune systems. The market is expanding as pharmaceutical companies intensify their efforts to develop effective RSV vaccines, which are critical for reducing hospitalizations and improving outcomes for at-risk groups. Several companies have advanced vaccine candidates, particularly focusing on innovative approaches such as mRNA technology. The approval of these new vaccines has generated considerable attention, leading to heightened investment in research and development.
Despite the growing market potential, challenges remain, particularly around market access, pricing, and distribution logistics. Vaccine adoption has also been influenced by evolving clinical guidelines and public health recommendations, which can affect demand. As more vaccines enter the market and further clinical data becomes available, competition in the RSV vaccine space is likely to increase, which could drive innovation and lower costs.
In the coming years, the market is expected to see sustained growth, with new vaccine approvals and expanded usage further contributing to the overall market size. This growth will be further supported by an increasing focus on preventative healthcare and efforts to address RSV-related healthcare burdens worldwide.
Respiratory Syncytial Virus Vaccines Market Size
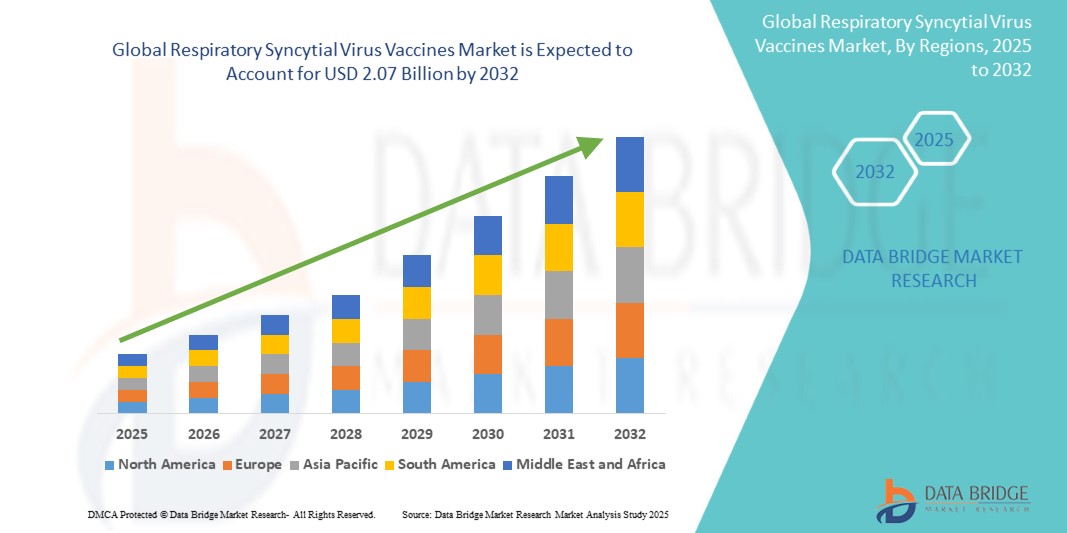
The global respiratory syncytial virus vaccines market size was valued at USD 1.47 billion in 2024 and is projected to reach USD 2.07 billion by 2032, with a CAGR of 4.40% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Respiratory Syncytial Virus Vaccines Market Trends
“Increasing Adoption of mRNA-based Vaccine Technology”
One key trend in the respiratory syncytial virus (RSV) vaccine market is the increasing adoption of mRNA-based vaccine technology. Following the success of mRNA vaccines in combating COVID-19, pharmaceutical companies have turned to this innovative technology to develop more effective RSV vaccines. mRNA vaccines work by instructing cells to produce a protein that triggers an immune response, offering a potentially quicker and more adaptable solution compared to traditional vaccine methods. Companies such as Moderna have been at the forefront of this trend, advancing their RSV vaccine candidates using mRNA technology, with successful trials and regulatory approvals creating strong market interest. This trend is significant because it not only accelerates the development of RSV vaccines but also enhances their precision and effectiveness. As mRNA technology continues to evolve, it is expected to play a pivotal role in the RSV vaccine market, driving future growth and innovation in preventing RSV infections.
Report Scope and Respiratory Syncytial Virus Vaccines Market Segmentation
|
Attributes |
Respiratory Syncytial Virus Vaccines Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America |
|
Key Market Players |
AstraZeneca (UK), AbbVie Inc. (U.S.), Alnylam Pharmaceuticals, Inc. (U.S.), Aimi Vaccine Co., Ltd. (China), Bausch Health Companies Inc. (Canada), Bavarian Nordic (Denmark), Enanta Pharmaceuticals, Inc. (U.S.), F. Hoffmann-La Roche Ltd (Switzerland), Gilead Sciences, Inc. (U.S.), GSK plc. (UK), Johnson & Johnson Services, Inc. (U.S.), Meissa Vaccines, Inc. (U.S.), Medivir AB (Sweden), Moderna, Inc. (U.S.), Merck & Co., Inc. (U.S.), Novavax (U.S.), Pfizer Inc. (U.S.), Sanofi (France), Sirnaomics (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Respiratory Syncytial Virus Vaccines Market Definition
Respiratory Syncytial Virus (RSV) vaccines are designed to protect individuals from infection by RSV, a common virus that causes respiratory illnesses, particularly in infants, the elderly, and people with weakened immune systems. These vaccines stimulate the immune system to produce antibodies that can recognize and neutralize the virus, preventing or reducing the severity of infection. RSV can lead to severe respiratory conditions, such as bronchiolitis and pneumonia, and is a major cause of hospitalization in young children. By vaccinating individuals, especially those at high risk, these vaccines aim to reduce the incidence of RSV-related hospitalizations, complications, and deaths. RSV vaccines are being developed using various technologies, including traditional methods and newer approaches such as mRNA, which have gained attention due to their success in the COVID-19 pandemic.
Respiratory Syncytial Virus Vaccines Market Dynamics
Drivers
- Increasing Burden of Respiratory Syncytial Virus Infections
One of the key drivers of the RSV vaccine market is the rising incidence of RSV-related infections, particularly among high-risk groups such as infants, the elderly, and immunocompromised individuals. RSV is a leading cause of respiratory illnesses such as bronchiolitis and pneumonia, which can result in hospitalization and, in severe cases, death. This growing burden has heightened the demand for preventive solutions such as vaccines. For instance, the approval of vaccines such as GSK’s Arexvy and Moderna's mRESVIA has addressed the increasing need for protection, especially in older adults. The prevalence of RSV-related diseases in vulnerable populations has emphasized the importance of vaccination. As awareness of RSV’s impact grows, the demand for vaccines has surged, directly contributing to market growth and expansion. The increasing number of approvals for RSV vaccines is expected to drive further market adoption and innovation.
- Advancements in Vaccine Technology
The development of new vaccine technologies, such as mRNA-based vaccines, has significantly driven the growth of the RSV vaccine market. Moderna's mRESVIA, which utilizes mRNA technology, is a prime instance of this advancement. The success of mRNA COVID-19 vaccines has accelerated interest in mRNA platforms for other infectious diseases, including RSV. mRNA vaccines offer faster production times and enhanced adaptability compared to traditional vaccine methods. This innovation has led to increased investor confidence and pharmaceutical companies' focus on RSV vaccine development. In addition, mRNA technology allows for more targeted immunity, potentially offering better efficacy. The approval of mRESVIA by the FDA in 2024 has been a pivotal moment in the market, signaling the viability of this platform for RSV prevention. This technological shift is expected to drive future growth by expanding the variety of available vaccines and improving overall effectiveness.
Opportunities
- Expansion of Vaccination Programs for High-Risk Populations
An emerging opportunity in the RSV vaccine market lies in expanding vaccination programs to target high-risk populations, including pregnant women, infants, the elderly, and individuals with compromised immune systems. With RSV posing a serious threat to infants and older adults, widespread vaccination programs could help prevent severe outcomes and reduce healthcare costs associated with hospitalizations. For instance, the FDA approval of GSK's Arexvy for individuals aged 60 and older in 2024 and Moderna's mRESVIA for adults 60+ have opened the door for large-scale vaccination initiatives. By expanding access to these vaccines, public health programs could significantly reduce RSV-related morbidity and mortality. As more health authorities incorporate RSV vaccines into routine immunization schedules for high-risk groups, the demand for these vaccines is expected to rise. This increased adoption in key populations is likely to fuel substantial market growth in the coming years.
- Integration of RSV Vaccines into Preventive Health Campaigns
Another significant opportunity for the RSV vaccine market lies in its integration into broader preventive health campaigns. Given the rising awareness of the importance of vaccination and disease prevention, integrating RSV vaccines into routine health check-ups or annual vaccination programs could drive market penetration. Companies can collaborate with healthcare organizations to create public health campaigns that emphasize the prevention of RSV, especially during peak seasons such as winter. For instance, vaccination drives targeted at infants and older adults can be linked with other flu or pneumonia prevention efforts. This integrated approach could increase the adoption of RSV vaccines, especially in regions with seasonal outbreaks. As preventive health strategies become a priority globally, integrating RSV vaccines into these initiatives could expand their reach, thereby boosting market growth by increasing vaccine uptake and accessibility to a broader population.
Restraints/Challenges
- Supply Chain and Distribution Challenges
A key restraint in the RSV vaccine market is the logistical challenges associated with vaccine distribution and supply chain management. Vaccines, particularly those using mRNA technology, often require strict storage conditions, such as refrigeration, which can complicate distribution in regions with limited infrastructure. For instance, Moderna’s mRESVIA, which requires cold chain handling, may face difficulties in reaching remote areas or lower-income countries where infrastructure for maintaining such conditions is lacking. In addition, production capacities may not always meet global demand, leading to delays or shortages. These challenges can prevent vaccines from reaching the populations that need them the most, limiting the overall impact of RSV vaccination campaigns. The added complexity of distribution could slow down the market’s growth, especially in regions where logistical challenges are more pronounced and access to vaccines is limited.
- Vaccine Hesitancy and Public Perception
A significant challenge in the RSV vaccine market is vaccine hesitancy, particularly among parents of infants and older adults, who may be concerned about the safety and necessity of vaccination. Despite the high risk of RSV infections leading to severe respiratory issues, some populations may not fully recognize the urgency of vaccination or may have concerns based on misinformation. This hesitancy can slow the adoption of newly approved vaccines, impacting market uptake. For instance, parents might prioritize vaccines they are more familiar with, such as the flu vaccine, while overlooking RSV prevention, even though it is a significant threat to infants. In addition, healthcare providers may not always prioritize RSV vaccinations, further limiting awareness.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Respiratory Syncytial Virus Vaccines Market Scope
The market is segmented on the basis of type, age group, and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Recombinant/ Conjugate/ Subunit
- mRNA
- Others
Age Group
- Pediatric
- Adults
Distribution Channel
- Hospital & Retail Pharmacies
- Government Suppliers
- Others
Respiratory Syncytial Virus Vaccines Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, type, age group, and distribution channel as referenced above.
The countries covered in the market report are U.S., Canada and Mexico, Germany, France, U.K., Italy, Russia, Spain, Denmark, Sweden, Norway, Rest of Europe, China, Japan, India, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Nigeria, Egypt, Kuwait, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America.
North America is expected to dominate the respiratory syncytial virus vaccines market. This is primarily due to the high prevalence of RSV infections among vulnerable populations such as infants, the elderly, and immunocompromised individuals in the region, along with increasing awareness about the virus and the benefits of vaccination. In addition, North America has well-established healthcare infrastructure, a high level of vaccine acceptance, and strong government support for vaccination programs, all of which contribute to the rapid adoption of new RSV vaccines.
Asia-Pacific is expected to exhibit the highest growth rate in the respiratory syncytial virus vaccines market. This growth is driven by factors such as increasing awareness of respiratory diseases, rising healthcare infrastructure improvements, and a growing aging population in countries such as Japan, China, and India. In addition, the rapid urbanization and improvement in healthcare access in emerging markets are expected to fuel demand for preventive healthcare solutions such as RSV vaccines.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Respiratory Syncytial Virus Vaccines Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Respiratory Syncytial Virus Vaccines Market Leaders Operating in the Market Are:
- AstraZeneca (UK)
- AbbVie Inc. (U.S.)
- Alnylam Pharmaceuticals, Inc. (U.S.)
- Aimi Vaccine Co., Ltd. (China)
- Bausch Health Companies Inc. (Canada)
- Bavarian Nordic (Denmark)
- Enanta Pharmaceuticals, Inc. (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Gilead Sciences, Inc. (U.S.)
- GSK plc. (UK)
- Johnson & Johnson Services, Inc. (U.S.)
- Meissa Vaccines, Inc. (U.S.)
- Medivir AB (Sweden)
- Moderna, Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- Novavax (U.S.)
- Pfizer Inc. (U.S.)
- Sanofi (France)
- Sirnaomics (U.S.)
Latest Developments in Respiratory Syncytial Virus Vaccines Market
- In October 2024, Pfizer Inc. announced that the U.S. Food and Drug Administration (FDA) had approved ABRYSVO (Respiratory Syncytial Virus Vaccine), a bivalent RSV prefusion F (RSVpreF) vaccine, for preventing lower respiratory tract disease (LRTD) caused by RSV in individuals aged 18 to 59 who are at higher risk for RSV-related LRTD. ABRYSVO now provides the broadest RSV vaccine indication for adults, expanding beyond the previous approval for those aged 60 and older
- In October 2024, Merck announced the presentation of positive results from the Phase 2b/3 clinical trial (MK-1654-004), which assessed clesrovimab, the company’s investigational prophylactic monoclonal antibody, designed to protect infants from respiratory syncytial virus (RSV) during their first RSV season
- In June 2024, GSK plc announced that the U.S. Food and Drug Administration (FDA) had approved Arexvy (Respiratory Syncytial Virus (RSV) Vaccine, Adjuvanted) for preventing RSV-related lower respiratory tract disease (LRTD) in adults aged 50 to 59 who are at increased risk
- In May 2024, Moderna, Inc. announced that the U.S. Food and Drug Administration (FDA) had approved mRESVIA (mRNA-1345), an mRNA-based respiratory syncytial virus (RSV) vaccine, to protect adults aged 60 and older from lower respiratory tract disease caused by RSV infection. This approval, granted under breakthrough therapy designation, marks the second mRNA product from Moderna to receive approval
- In May 2023, GSK plc announced that the U.S. Food and Drug Administration (FDA) had approved Arexvy (respiratory syncytial virus vaccine, adjuvanted) for preventing lower respiratory tract disease (LRTD) caused by respiratory syncytial virus (RSV) in individuals aged 60 and older. This marks the first approval of an RSV vaccine for older adults worldwide
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.