Global Resuscitation Consumables Market
Market Size in USD Billion
CAGR :
% 
 USD
1.60 Billion
USD
2.70 Billion
2024
2032
USD
1.60 Billion
USD
2.70 Billion
2024
2032
| 2025 –2032 | |
| USD 1.60 Billion | |
| USD 2.70 Billion | |
|
|
|
|
Resuscitation Consumables Market Analysis
The global resuscitation consumables market is evolving with innovations in medical devices designed to aid in emergency situations such as cardiac arrest or respiratory failure. Advances in technology have led to the development of more effective, reliable, and user-friendly resuscitation tools. For instance, portable defibrillators now feature automated functions that simplify their use in emergency situations, improving patient survival rates. Additionally, airway management devices are being enhanced with smart technologies that allow real-time monitoring of a patient’s airway, increasing the efficacy of interventions. Another major innovation is the development of advanced ventilators that offer more precise control of air delivery, ensuring better patient outcomes in critical situations.
The market growth is driven by the rising incidences of sudden cardiac arrests, respiratory conditions, and the increasing need for life-saving interventions. As healthcare facilities expand and the focus on emergency care intensifies, demand for resuscitation consumables is expected to rise significantly.
Resuscitation Consumables Market Size
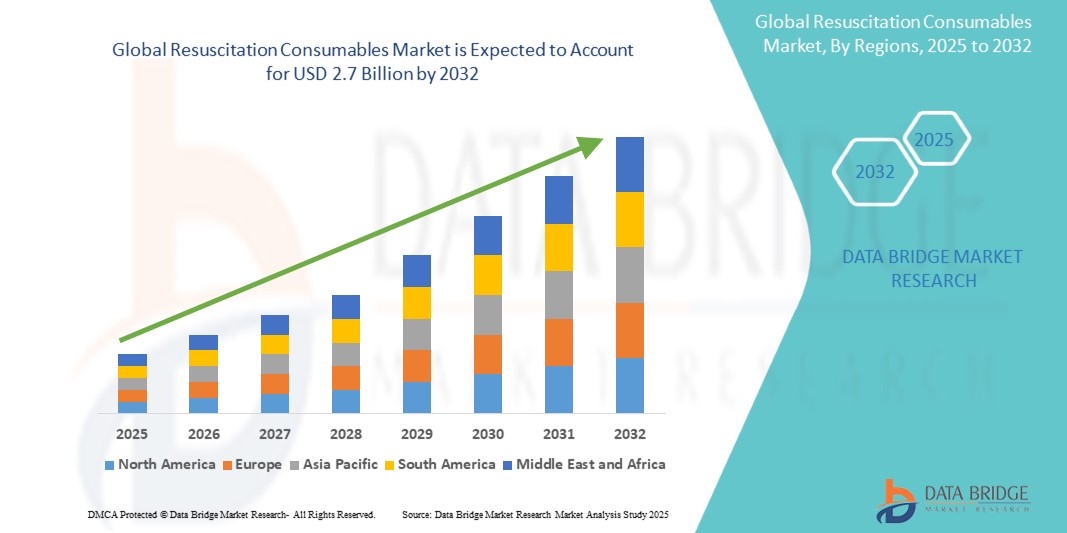
The global resuscitation consumables market size was valued at USD 1.6 billion in 2024 and is projected to reach USD 2.7 billion by 2032, with a CAGR of 6.90% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Resuscitation Consumables Market Trends
"Integration of Smart Technology in Resuscitation Devices"
The integration of smart technology into resuscitation devices is transforming emergency medical care. Devices like automated external defibrillators (AEDs) now incorporate cloud connectivity and artificial intelligence (AI), allowing for real-time data analysis and remote support. This means healthcare professionals can receive instant feedback and guidance during resuscitation efforts, ensuring more accurate decision-making and better outcomes for patients. Similarly, ventilators are being enhanced with adaptive features that adjust to a patient’s specific respiratory needs, improving performance and efficiency in critical situations. These innovations not only help improve patient survival rates but also make resuscitation processes more efficient and precise. As a result, the resuscitation consumables market is experiencing significant growth, driven by the increasing demand for advanced, technology-driven medical devices that offer enhanced functionality and improved patient care during emergencies. The continuous evolution of these smart technologies promises to revolutionize life-saving practices in both hospital and out-of-hospital settings.
Report Scope and Resuscitation Consumables Market Segmentation
|
Attributes |
Resuscitation Consumables Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America |
|
Key Market Players |
Medtronic (Ireland), Koninklijke Philips (Netherlands), Zoll Medical Corporation (U.S.), Stryker Corporation (U.S.), GE Healthcare (U.S.), Smiths Medical, Inc. (U.K.), 3M (U.S.), Ambu A/S (Denmark), Cardinal Health, Inc. (U.S.), Drägerwerk AG & Co. KGaA (Germany), Flexicare (Group) Limited (U.K.), General Electric Company (U.S.), Intersurgical Ltd. (U.K.), Koninklijke Philips N.V. (Netherlands), Nihon Kohden Corporation (Japan), ResMed (U.S.), Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (China), ICU Medical, Inc. (U.S.), Teleflex Incorporated (U.S.), Becton, Dickinson and Company (BD) (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Resuscitation Consumables Market Definition
Resuscitation consumables are medical devices and equipment used to revive individuals experiencing life-threatening conditions such as cardiac arrest or respiratory failure. These devices are critical in emergency medical settings, providing immediate intervention to sustain life. Products such as defibrillators, airway management tools, and ventilators are designed to be used quickly and efficiently by healthcare providers in various situations. With advancements in technology, many of these devices are now equipped with automation and smart features that improve their effectiveness during emergencies.
Resuscitation Consumables Market Dynamics
Drivers
• Growing Demand for Emergency Medical Services
The increasing demand for emergency medical services is illustrated by the rising number of cardiac arrests and trauma-related emergencies worldwide. For example, in the United States, approximately 350,000 people experience out-of-hospital cardiac arrests annually, with defibrillators playing a critical role in survival rates. In response, healthcare systems and EMS providers are increasingly relying on resuscitation consumables such as automated external defibrillators (AEDs), portable ventilators, and CPR kits. The growing recognition of the need for rapid emergency intervention is fueling the demand for these life-saving consumables globally.
- Advancements in Medical Technology
Technological advancements, such as the development of portable defibrillators like the Philips HeartStart FR3, are making it easier to provide immediate care in emergency situations. These devices feature real-time feedback mechanisms to ensure optimal CPR quality, enhancing the survival rate of cardiac arrest patients. Additionally, advanced airway management devices such as the i-gel supraglottic airway, which is quicker and easier to insert compared to traditional intubation, are gaining traction in emergency care settings. These innovations in medical technology are helping to improve the effectiveness of resuscitation efforts and are driving the global demand for these consumables.
Opportunities
- Growing Prevalence of Cardiac Diseases
The increasing prevalence of cardiac diseases is evident with the World Health Organization (WHO) reporting that cardiovascular diseases are the leading cause of death worldwide, accounting for 32% of all global deaths. This has led to an increasing demand for resuscitation consumables, especially in high-risk populations. For instance, hospitals in countries like India, which are witnessing a rise in lifestyle-related cardiovascular conditions, are expanding their emergency medical services and adopting advanced resuscitation technologies, such as automated defibrillators and advanced airway management devices, to manage cardiac emergencies effectively.
- Expansion of Emergency Medical Services in Emerging Markets
In emerging markets like China and Brazil, the expansion of emergency medical services is becoming a priority. For example, in China, the government has invested in improving emergency healthcare infrastructure, which includes the distribution of AEDs in public spaces and training emergency personnel to use advanced resuscitation equipment. This has created new opportunities for resuscitation consumables companies to enter these growing markets, which are experiencing increased demand for life-saving medical devices.
Restraints/Challenges
- High Cost of Advanced Resuscitation Equipment
The high cost of advanced resuscitation equipment is a significant challenge for the market. For example, the cost of an AED like the Philips HeartStart OnSite AED can range from $1,200 to $1,500, while advanced ventilators like the Hamilton C1 can cost upwards of $20,000. In countries with limited healthcare budgets, such as parts of Sub-Saharan Africa, the expense of purchasing and maintaining such equipment can be prohibitive. In rural areas of India, many smaller hospitals and clinics cannot afford the initial investment in these high-quality devices, which limits their ability to provide essential emergency care. As a result, these regions often rely on older or less effective equipment, which may not meet international standards, further hindering the effectiveness of emergency medical services and contributing to poorer patient outcomes. This financial barrier slows the adoption of critical resuscitation technologies in low-resource settings.
- Regulatory and Compliance Challenges
The regulatory challenges are seen in markets like the European Union, where medical devices must meet rigorous standards set by the European Medicines Agency (EMA) and other national health authorities. Manufacturers often face delays in obtaining necessary certifications, impacting the speed with which new resuscitation technologies can be introduced to the market. A notable example is the prolonged approval process for new medical devices, such as advanced defibrillators and ventilators, which must undergo extensive clinical trials, regulatory reviews, and compliance assessments before they can be marketed and sold globally.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Resuscitation Consumables Market Scope
The market is segmented on the basis of product, usage, end user, distribution channel, and patient type. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product
- Defibrillators
- Automated External Defibrillators (AEDs)
- Implantable Cardioverter Defibrillators (ICDs)
- Manual Defibrillators
- Ventilators
- Non-invasive Ventilators
- Invasive Ventilators
- Portable Ventilators
- Airway Management Devices
- Endotracheal Tubes
- Laryngeal Mask Airways (LMAs)
- Oropharyngeal and Nasopharyngeal Airways
- Tracheostomy Tubes
- Others
- CPR Masks and Barriers
- Oxygen Masks and Cannulas
- Suction Devices
- Rescue Breathing Equipment
- Resuscitation Bags
Usage
- Disposable
- Reusable
End User
- Hospitals
- Clinics
- Ambulance Services
- Others (Home Care, Nursing Homes, etc.)
Distribution Channel
- Direct Sales
- Retail Sales
Patient Type
- Adult Patients
- Pediatric Patients
Resuscitation Consumables Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, product, usage, end user, distribution channel, and patient type as referenced above.
The countries covered in the market report are U.S., Canada and Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa, Brazil, Argentina and Rest of South America.
North America is expected to dominate the resuscitation consumables market due to the significant increase in research and development investments, along with the growing number of government initiatives to enhance healthcare. The region benefits from advanced healthcare infrastructure, which drives demand for sophisticated diagnostic tools such as clinical microscopes. In addition, the presence of leading manufacturers and continuous technological advancements further support North America's leadership in the market. This growth is further fueled by the rising healthcare awareness and medical needs.
Asia-Pacific is expected to exhibit the highest growth rate in the resuscitation consumables market during the forecast period due to increasing government expenditure on the healthcare sector. The region's rising technological advancements and government initiatives to improve healthcare infrastructure are key drivers of market growth. In addition, growing investments in medical research and healthcare facilities in countries such as China, India, and Japan are further boosting the demand for clinical microscopes in the region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Resuscitation Consumables Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Resuscitation Consumables Market Leaders Operating in the Market Are:
- Medtronic (Ireland)
- Koninklijke Philips (Netherlands)
- Zoll Medical Corporation (U.S.)
- Stryker Corporation (U.S.)
- GE Healthcare (U.S.)
- Smiths Medical, Inc. (U.K.)
- 3M (U.S.)
- Ambu A/S (Denmark)
- Cardinal Health, Inc. (U.S.)
- Drägerwerk AG & Co. KGaA (Germany)
- Flexicare (Group) Limited (U.K.)
- General Electric Company (U.S.)
- Intersurgical Ltd. (U.K.)
- Koninklijke Philips N.V. (Netherlands)
- Nihon Kohden Corporation (Japan)
- ResMed (U.S.)
- Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (China)
- ICU Medical, Inc. (U.S.)
- Teleflex Incorporated (U.S.)
- Becton, Dickinson and Company (BD) (U.S.)
Latest Developments in Resuscitation Consumables Market
- In December 2024, Baptist Health Paducah has been honored with the 2024 Lighthouse Award for its innovative project, "Staying Alive: Utilizing Technology to Improve Resuscitation Quality." The initiative focused on enhancing CPR quality across the hospital, improving teamwork, proficiency, and CPR skills among staff, and ultimately increasing patient survival rates. Over three years, survival rates improved from 60% to 80%, and discharge rates from 21% to 40%. This recognition highlights Baptist Health's commitment to advancing healthcare quality and patient safety
- In July 2023, Ambu has announced the commercialization of its latest solution for intubation and one lung ventilation (OLV) procedures, the Ambu VivaSight 2 SLT (single-lumen tube), in Europe. This launch completes Ambu's OLV portfolio, offering a comprehensive range of products, including single- and double-lumen tubes with integrated cameras, an endobronchial blocker tube, single-use bronchoscopes, and a full-HD endoscopy display system. The VivaSight 2 SLT provides clinicians with enhanced tube placement visualization, offering flexibility in choosing the best solution for each procedure
- In April 2023, Nordic Capital has acquired a majority stake in corpuls, a leading med-tech company specializing in emergency medical solutions, including defibrillators and monitoring systems. The partnership aims to support corpuls' growth, innovation, and expansion into new markets and the hospital segment
- In March 2021, a new device, the SARUS-CPR hood, developed by Keela Outdoors, NHS Tayside, and Scottish Health Innovations (SHIL), aims to revolutionize resuscitation for first responders. This lightweight, transparent fabric hood creates a barrier between the patient and responder, reducing the risk of infection from bacteria and viruses like COVID-19. Designed for quick application, it enhances safety and efficiency during airway ventilation in emergency settings such as hospitals and ambulances
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.