Global Rift Valley Fever Rvf Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
2.47 Billion
USD
3.90 Billion
2024
2032
USD
2.47 Billion
USD
3.90 Billion
2024
2032
| 2025 –2032 | |
| USD 2.47 Billion | |
| USD 3.90 Billion | |
|
|
|
|
Rift Valley Fever (RVF) Treatment Market Size
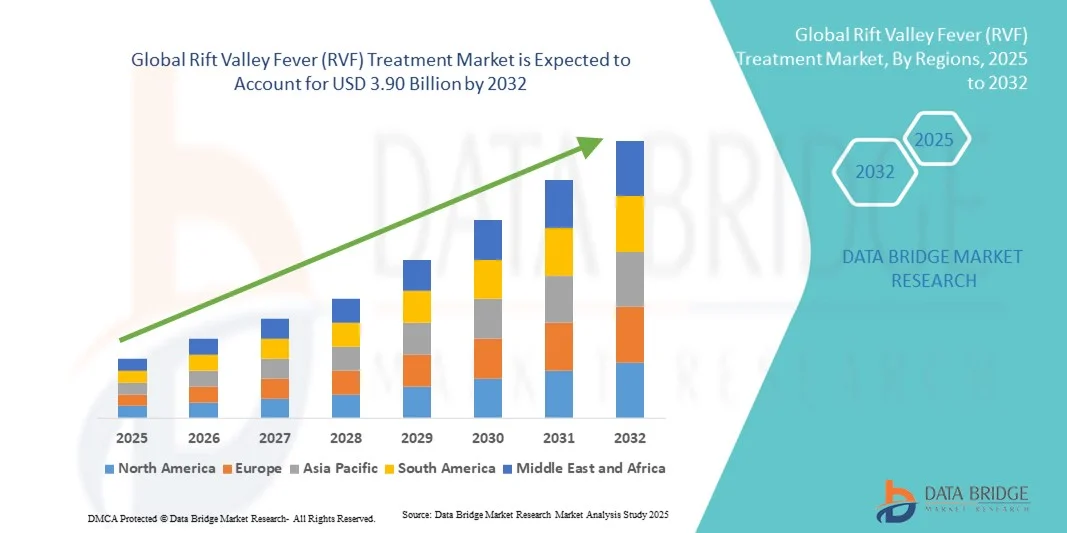
- The global rift valley fever (RVF) treatment market size was valued at USD 2.47 billion in 2024 and is expected to reach USD 3.90 billion by 2032, at a CAGR of 5.90% during the forecast period
- The market growth is largely fueled by the increasing prevalence of rift valley fever (RVF) in livestock and human populations, coupled with rising awareness about preventive measures and timely treatment interventions in endemic regions
- Furthermore, growing investment in vaccine development, antiviral therapies, and supportive care solutions, along with expanding healthcare infrastructure and veterinary services, is accelerating the uptake of rift valley fever (RVF) treatment solutions, thereby significantly boosting the industry’s growth
Rift Valley Fever (RVF) Treatment Market Analysis
- The Rift Valley Fever (RVF) Treatment market is witnessing significant growth, driven by the increasing prevalence of RVF outbreaks in livestock and humans, and the rising demand for effective antiviral therapies, vaccines, and supportive care measures in endemic regions
- The escalating demand for RVF treatment is primarily fueled by growing awareness among healthcare providers and veterinary services, ongoing research and development of vaccines and antivirals, and expanding healthcare and veterinary infrastructure
- North America dominated the rift valley fever (RVF) treatment market with the largest revenue share of 41.2% in 2024, driven by advanced healthcare and veterinary infrastructure, strong research initiatives, and the presence of key industry players developing preventive and therapeutic solutions
- Asia-Pacific is expected to be the fastest-growing region in the rift valley fever (RVF) treatment market during the forecast period, with a projected CAGR due to increasing investments in healthcare, growing awareness of RVF prevention, and expanding veterinary and public health services in countries such as China, India, and Australia
- The Supportive Therapy segment dominated the market with a revenue share of 72.4% in 2024, driven by the lack of disease-specific antiviral drugs and the widespread adoption of symptom management strategies
Report Scope and Rift Valley Fever (RVF) Treatment Market Segmentation
|
Attributes |
Rift Valley Fever (RVF) Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Rift Valley Fever (RVF) Treatment Market Trends
Increasing Focus on Early Diagnosis and Preventive Care
- A notable trend in the RVF treatment market is the rising emphasis on early diagnosis through rapid testing and preventive interventions in both human and livestock populations
- Governments and healthcare organizations are increasingly implementing surveillance programs to detect RVF outbreaks early, enabling prompt treatment and reducing mortality rates
- For instance, in September 2023, Kenya’s Ministry of Health launched a nationwide RVF surveillance and early-warning program integrating mobile-based reporting systems for healthcare workers
- Early detection allows timely administration of antiviral therapy, supportive care, and preventive measures, enhancing patient outcomes
- Veterinary interventions, including livestock vaccination, complement human treatment efforts and reduce disease transmission
- Awareness campaigns educate communities on recognizing RVF symptoms and seeking treatment promptly
- Integration of local clinics with regional hospitals ensures rapid patient referral and care
- Public-private partnerships in healthcare infrastructure development support diagnostic and treatment accessibility
- Research initiatives on rapid diagnostic kits contribute to faster disease detection
- Data-driven epidemiological models help forecast outbreaks, informing healthcare readiness
- The trend toward early intervention and preventive care is reshaping RVF management strategies globally
Rift Valley Fever (RVF) Treatment Market Dynamics
Driver
Rising Prevalence and Enhanced Healthcare Awareness
- The increasing incidence of RVF in Africa, the Middle East, and other endemic regions is a primary driver for the market
- Awareness programs by governments and NGOs have improved early recognition and treatment adoption
- For instance, in April 2024, WHO issued updated treatment guidelines emphasizing early antiviral therapy and supportive care for RVF cases in outbreak regions
- Hospitals and specialty clinics are stocking antiviral drugs and supportive care medications
- Adoption of guideline-based therapy ensures standardized and effective patient management
- Rising livestock infections drive prophylactic and therapeutic interventions. Investments in healthcare infrastructure and personnel training increase treatment availability. Clinical studies demonstrating the effectiveness of existing antiviral therapies support wider adoption
- International funding initiatives enhance program implementation and drug access. Community awareness campaigns indirectly boost demand for treatment. Research on novel therapeutics continues to expand treatment options
- The cumulative effect of rising prevalence, awareness, and healthcare readiness accelerates market growth
Restraint/Challenge
Limited Drug Access and High Treatment Costs
- Restricted availability of approved antiviral drugs and specialized care facilities limits treatment adoption. High cost of treatment and insufficient healthcare infrastructure pose barriers, especially in remote areas
- For instance, in February 2023, reports from Sudan highlighted treatment shortages during RVF outbreaks due to logistical challenges and high drug prices
- Dependence on hospital-based care restricts access during peak outbreaks. Regulatory delays in novel drug approvals affect market penetration
- Low insurance coverage and public funding limitations reduce affordability. Supply chain inconsistencies can create drug shortages
- Lack of standardized protocols in some regions causes delayed therapy. Adverse effects of certain antivirals may limit use in vulnerable populations
- Awareness gaps among healthcare providers can delay intervention
- Cold-chain and storage logistics in tropical climates complicate distribution
- Overcoming these challenges requires targeted investment, robust supply chains, and cost-effective therapy options
Rift Valley Fever (RVF) Treatment Market Scope
The market is segmented on the basis of treatment, end user, and distribution channel.
- By Treatment
On the basis of treatment, the Rift Valley Fever (RVF) Treatment market is segmented into Supportive Therapy and Others. The Supportive Therapy segment dominated the market with a revenue share of 72.4% in 2024, driven by the lack of disease-specific antiviral drugs and the widespread adoption of symptom management strategies. Hospitals and clinics prefer supportive care to stabilize patients, including fluid replacement, pain management, and monitoring of liver and kidney function. Availability of guidelines from WHO and CDC ensures standardized treatment protocols. High prevalence of moderate to severe RVF cases in endemic regions strengthens adoption. Supportive therapy is utilized across both adult and pediatric populations. Integration into routine hospital care and public health programs increases market share. Government and NGO initiatives supporting basic care further consolidate dominance. Availability of intravenous fluids, analgesics, and antipyretics supports broad implementation. Training healthcare professionals in supportive therapy protocols ensures consistent delivery. Supply chain accessibility in endemic areas ensures therapy continuity. The segment benefits from established hospital practices and community health awareness. Combination with monitoring and follow-up services reinforces revenue generation.
The Others segment is expected to witness the fastest CAGR of 9.2% from 2025 to 2032, driven by experimental treatments, novel antiviral drugs, and adjunct therapies under clinical investigation. Increased investment in R&D for RVF-specific therapies supports growth. Emerging biopharmaceutical products are gradually entering the market. Early adoption by specialty clinics and research hospitals accelerates uptake. Expansion into emerging markets with high disease prevalence contributes to growth. Government and NGO support for clinical trials increases access. Awareness campaigns about advanced treatment options drive adoption. Collaboration between research institutes and hospitals facilitates pilot programs. Improved regulatory pathways enable faster approval of experimental drugs. Increasing availability of combination therapies enhances effectiveness. Rising physician knowledge and guideline updates support uptake. Demand for alternatives beyond supportive care drives market expansion.
- By End User
On the basis of end user, the Rift Valley Fever (RVF) Treatment market is segmented into Hospital, Clinics, Ambulatory Surgical Centers, and Others. The Hospital segment dominated the market with a revenue share of 65.7% in 2024, due to comprehensive treatment facilities, access to trained healthcare personnel, and availability of supportive therapies. Hospitals manage severe cases requiring intensive monitoring, intravenous therapy, and multi-disciplinary care. High patient throughput and referral networks enhance adoption. Government and international health programs prioritize hospitals for RVF management. Presence of emergency and infectious disease units ensures timely treatment. Hospitals are equipped with laboratory and diagnostic support to guide therapy. Integration with national reporting systems strengthens coordination. Hospitals provide both adult and pediatric care. Access to first-line supportive therapies increases patient outcomes. Insurance coverage and funding for treatment programs further support revenue. Research and pilot programs within hospitals promote treatment adoption. Hospitals serve as primary hubs for clinical studies and training healthcare staff.
The Clinics segment is expected to witness the fastest CAGR of 8.5% from 2025 to 2032, driven by outpatient care, early detection initiatives, and management of mild to moderate RVF cases. Clinics expand access to rural and semi-urban populations. Adoption is supported by community awareness campaigns. Mobile and telemedicine services enhance patient monitoring. Clinics provide rapid treatment initiation, reducing disease complications. Growth in specialized infectious disease clinics supports segment expansion. Public-private partnerships enable better resource allocation. Clinics increasingly participate in government-funded RVF programs. Training staff in supportive care protocols improves service quality. Expansion into high-prevalence regions accelerates adoption. Access to basic medications and follow-up care ensures patient compliance. Integration with regional hospitals for severe cases enhances credibility. Clinics’ flexible service offerings cater to growing outpatient demand.
- By Distribution Channel
On the basis of distribution channel, the Rift Valley Fever (RVF) Treatment market is segmented into Hospital Pharmacy, Retail Pharmacy, and Online Pharmacy. The Hospital Pharmacy segment dominated the market with a revenue share of 58.3% in 2024, due to direct integration with hospital treatment protocols and immediate access to essential supportive medications. Hospitals stock standard therapies, ensuring timely treatment. Hospital pharmacies benefit from bulk procurement and government subsidies. Staffed with trained pharmacists, they provide counseling and dosage management. High patient footfall reinforces demand. Hospitals ensure adherence to treatment guidelines. Direct supply chains reduce stockouts. Integration with patient monitoring supports therapy continuity. Inclusion of both adult and pediatric formulations strengthens adoption. Hospitals’ capacity for IV medications increases market share. Partnerships with NGOs enhance medication availability. Hospitals manage emergency and outbreak situations effectively.
The Online Pharmacy segment is expected to witness the fastest CAGR of 10.1% from 2025 to 2032, driven by increased internet penetration, telemedicine consultations, and patient preference for home delivery of supportive care medications. Online channels expand reach to rural and remote areas. Convenience and accessibility accelerate adoption. Collaboration with healthcare providers ensures prescription validation. Emerging markets adoption is increasing. Growth in mobile apps and e-pharmacy platforms supports ordering and tracking. Availability of both oral and parenteral medications enhances uptake. Patient education and counseling through online platforms improve compliance. COVID-19 highlighted the importance of remote access to treatment. Partnerships with logistics providers ensure timely delivery. Online pharmacies offer cost-effective access to essential therapies. Digital promotion and awareness campaigns drive visibility. Regulatory support for telehealth and e-pharmacy models fuels segment growth.
Rift Valley Fever (RVF) Treatment Market Regional Analysis
- North America dominated the rift valley fever (RVF) treatment market with the largest revenue share of 41.2% in 2024
- Driven by advanced healthcare and veterinary infrastructure, strong research initiatives, and the presence of key industry players developing preventive and therapeutic solutions
- The market remains the primary contributor to regional growth due to early adoption of innovative vaccines, antiviral drugs, and supportive care protocols
U.S. Rift Valley Fever (RVF) Treatment Market Insight
The U.S. rift valley fever (RVF) treatment market captured the largest revenue share in North America in 2024, fueled by increasing awareness of RVF management, availability of advanced therapeutics, and the adoption of standardized preventive and treatment guidelines in hospitals, clinics, and veterinary centers. Government-supported surveillance programs and public health campaigns further drive market expansion.
Europe Rift Valley Fever (RVF) Treatment Market Insight
The Europe rift valley fever (RVF) treatment market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by stringent veterinary regulations, the need for effective disease control in livestock, and the rising demand for preventive therapeutics. European countries are increasingly investing in research programs for vaccines and antiviral therapies, supporting market growth across both animal and human healthcare segments.
U.K. Rift Valley Fever (RVF) Treatment Market Insight
The U.K rift valley fever (RVF) treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by investments in veterinary health, the growing focus on preventive strategies, and rising awareness among healthcare professionals. Collaborative initiatives with research institutions and enhanced diagnostic capabilities are expected to support market development.
Germany Rift Valley Fever (RVF) Treatment Market Insight
The Germany rift valley fever (RVF) treatment market is expected to expand at a considerable CAGR during the forecast period, fueled by strong healthcare infrastructure, active R&D in therapeutic solutions, and the demand for effective preventive and treatment strategies. Germany’s emphasis on technological innovation and sustainability promotes adoption of advanced RVF treatments in both human and veterinary healthcare sectors.
Asia-Pacific Rift Valley Fever (RVF) Treatment Market Insight
The Asia-Pacific rift valley fever (RVF) treatment market is poised to grow at the fastest CAGR during the forecast period, driven by increasing healthcare and veterinary investments, rising disposable incomes, and growing public and livestock health awareness in countries such as China, India, and Australia. Enhanced government initiatives promoting disease prevention and the expansion of diagnostic and treatment facilities are major growth drivers.
Japan Rift Valley Fever (RVF) Treatment Market Insight
The Japan rift valley fever (RVF) treatment market is gaining momentum due to the country’s advanced healthcare system, high patient and veterinary awareness, and focus on preventive care. Hospitals, specialty clinics, and veterinary centers emphasizing early detection and timely intervention further support market growth, with research programs enhancing the availability of effective treatments.
China Rift Valley Fever (RVF) Treatment Market Insight
The China rift valley fever (RVF) treatment market accounted for the largest market revenue share in Asia-Pacific in 2024, attributed to expanding healthcare and veterinary infrastructure, increasing number of hospitals and specialty clinics, rising prevalence of RVF, and growing public and livestock awareness regarding effective preventive and therapeutic options. Government initiatives supporting research and disease monitoring are also key contributors to market expansion.
Rift Valley Fever (RVF) Treatment Market Share
The Rift Valley Fever (RVF) Treatment industry is primarily led by well-established companies, including:
• Pfizer Inc. (U.S.)
• Merck & Co., Inc. (U.S.)
• Sanofi (France)
• GSK plc (U.K.)
• Johnson & Johnson and its affiliates (U.S.)
• Boehringer Ingelheim GmbH (Germany)
• Bayer AG (Germany)
• Novartis AG (Switzerland)
• BioCryst Pharmaceuticals, Inc. (U.S.)
• Emergent BioSolutions Inc. (U.S.)
• Inovio Pharmaceuticals, Inc. (U.S.)
• Valneva SE (France)
• Bavarian Nordic A/S (Denmark)
• Ceva Santé Animale (France)
• Zoetis Inc. (U.S.)
Latest Developments in Global Rift Valley Fever (RVF) Treatment Market
- In July 2025, the Coalition for Epidemic Preparedness Innovations (CEPI) initiated the first Phase II clinical trial of a human RVF vaccine in Kenya. This trial, conducted by the KEMRI-Wellcome Trust Research Programme in Kilifi, represents the most advanced stage of RVF vaccine testing in an outbreak-prone region to date. The ChAdOx1 RVF vaccine candidate, developed by Oxford University, aims to assess safety and immunogenicity in humans
- In June 2024, CEPI organized a workshop in Nairobi to discuss RVF epidemiology and modeling, aiming to inform human vaccine development. The meeting brought together experts to evaluate disease burden, transmission dynamics, and the feasibility of large-scale clinical trials in East Africa
- In May 2025, researchers published a study on a DNA-based veterinary RVF vaccine candidate that demonstrated protective efficacy in sheep under natural field conditions. The vaccine, encoding a consensus RVFV glycoprotein precursor, showed promise in preventing disease transmission and could be a valuable tool for livestock protection
- In October 2024, CEPI announced funding for collaborative research projects in Kenya and Tanzania to strengthen scientific understanding of RVF and guide future clinical trials for human vaccine candidates. These studies aim to map the extent of RVF's impact and inform vaccine development strategies
- In 2023, a review published in Nature highlighted the progress in RVF vaccine development, noting several veterinary vaccines have been licensed in endemic countries, while human vaccines remain in early-stage development. The review emphasized the need for continued research to address the lack of licensed human vaccines
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.