Global Rna Based Cardiac Therapeutics Market
Market Size in USD Billion
CAGR :
% 
 USD
792.00 Billion
USD
2,543.26 Billion
2024
2032
USD
792.00 Billion
USD
2,543.26 Billion
2024
2032
| 2025 –2032 | |
| USD 792.00 Billion | |
| USD 2,543.26 Billion | |
|
|
|
|
RNA-Based Cardiac Therapeutics Market Size
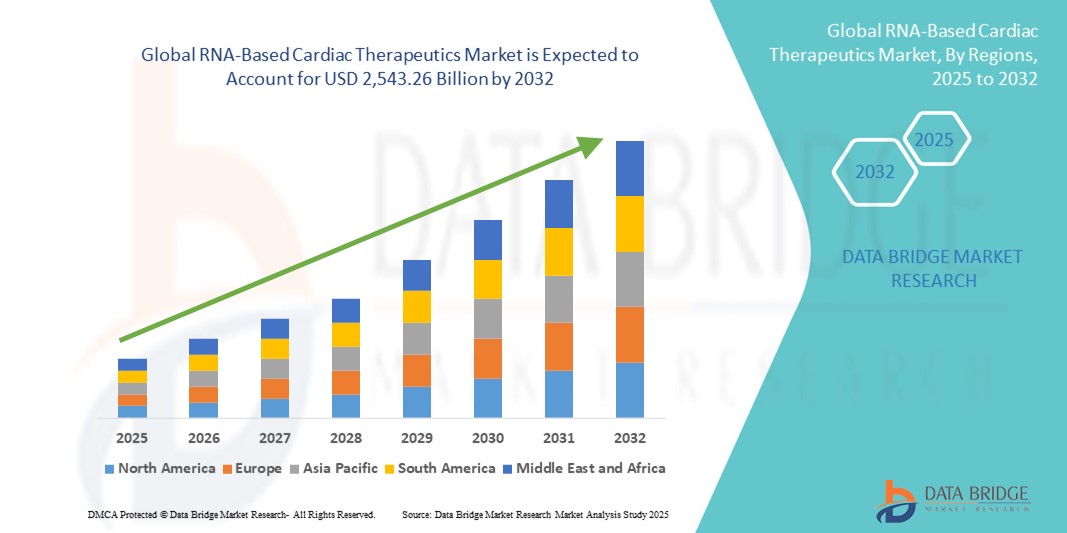
- The global RNA-based cardiac therapeutics market size was valued at USD billion in 2024 and is expected to reach USD 2,543.26 billion by 2032, at a CAGR of 15.70% during the forecast period
- The market growth is largely driven by advances in RNA technologies and the increasing prevalence of cardiovascular diseases worldwide, prompting a shift toward precision medicine and targeted therapies
- Furthermore, rising investments in RNA-based drug development, combined with favorable regulatory support and growing clinical success rates, are positioning RNA therapeutics as a transformative solution in cardiac care. These converging factors are accelerating the adoption of RNA-based cardiac treatments, thereby significantly boosting the industry's growth
RNA-Based Cardiac Therapeutics Market Analysis
- RNA-based cardiac therapeutics, leveraging modalities such as siRNA, antisense oligonucleotides, and mRNA, are emerging as promising treatments for various cardiovascular conditions due to their precision targeting, gene-silencing capabilities, and potential for disease modification at the molecular level
- The accelerating demand for RNA-based cardiac therapies is primarily fueled by the rising global burden of cardiovascular diseases, increasing investment in RNA therapeutics, and growing clinical evidence supporting the efficacy and safety of RNA-targeted interventions in heart-related disorders
- North America dominated the RNA-based cardiac therapeutics market with the largest revenue share of 43.3% in 2024, characterized by advanced healthcare infrastructure, strong R&D funding, early regulatory approvals, and the presence of leading biotech companies pioneering RNA innovation, with the U.S. seeing rapid clinical advancements in RNA drugs targeting heart failure, hyperlipidemia, and arrhythmias
- Asia-Pacific is expected to be the fastest growing region in the RNA-based cardiac therapeutics market during the forecast period due to increasing cardiovascular disease prevalence, improved healthcare access, and expanding biotech investments
- The antisense oligonucleotide segment dominated the RNA-based cardiac therapeutics market with a market share of 40% in 2024, driven by its well-established mechanism of action, scalability, and ongoing clinical trials targeting cardiac gene expressions
Report Scope and RNA-Based Cardiac Therapeutics Market Segmentation
|
Attributes |
RNA-Based Cardiac Therapeutics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
RNA-Based Cardiac Therapeutics Market Trends
Advancing Precision Cardiology Through RNA Modulation
- A significant and accelerating trend in the global RNA-based cardiac therapeutics market is the evolution of precision medicine through RNA technologies such as antisense oligonucleotides (ASOs), small interfering RNA (siRNA), and mRNA. These modalities are reshaping cardiovascular treatment by enabling gene-specific targeting and regulation, offering therapeutic potential for previously untreatable or genetically complex cardiac conditions
- For instance, Ionis Pharmaceuticals and Novartis are advancing RNA-targeted therapies such as pelacarsen (targeting Lp(a)) and eplontersen (targeting transthyretin amyloidosis), both aimed at modifying cardiovascular disease risk at a molecular level. Similarly, CureVac and Moderna are exploring mRNA platforms for cardiac regeneration
- RNA-based interventions offer tailored mechanisms of action, including silencing pathogenic gene expression or restoring protein function, and are increasingly being designed to reduce off-target effects while enhancing cardiac tissue uptake. These therapies can address genetic dyslipidemias, heart failure, and cardiomyopathies—especially in cases where traditional drugs are ineffective or contraindicated
- Innovations in delivery systems such as GalNAc-conjugated RNA, lipid nanoparticles, and tissue-specific ligands are enabling more efficient and targeted therapeutic delivery to the heart and liver, addressing one of the key challenges in RNA drug development
- This trend toward disease-modifying and gene-directed RNA therapeutics is fundamentally transforming the landscape of cardiac care. Companies such as Silence Therapeutics, Alnylam, and Cardior Pharmaceuticals are increasingly investing in cardiovascular RNA candidates, reflecting strong market confidence in this next-generation modality
- The demand for RNA-based cardiac therapeutics is growing rapidly across both rare and prevalent cardiac diseases, as clinicians and healthcare systems seek innovative, long-lasting treatments with a genetic or molecular basis for action
RNA-Based Cardiac Therapeutics Market Dynamics
Driver
Rising Cardiovascular Disease Burden and Innovation in RNA Therapeutics
- The escalating global burden of cardiovascular diseases (CVDs), which remain the leading cause of mortality worldwide, is a key driver behind the growing demand for RNA-based therapeutic innovations in cardiology
- For instance, more than 17.9 million people die annually due to CVDs, prompting a shift toward novel, molecular-level therapies. RNA-based drugs, by addressing genetic risk factors such as elevated Lp(a) or familial hypercholesterolemia, offer unique clinical advantages not addressed by conventional medications
- The rapid progress in RNA drug platforms validated through COVID-19 vaccine success—has spurred increased investment and regulatory support in applying these platforms to chronic diseases, including cardiac disorders
- Key companies such as Ionis, Alnylam, and Cardior Pharmaceuticals are actively expanding their cardiovascular RNA pipelines, supported by favorable clinical trial outcomes and strategic partnerships with pharma giants
- The ability to tailor RNA molecules for specific cardiac gene targets offers promising therapeutic value in areas such as heart failure, arrhythmias, and myocardial remodeling, with several candidates currently in Phase II/III trials. Growing healthcare funding, precision medicine initiatives, and improved RNA stability technologies further contribute to the market’s momentum
Restraint/Challenge
Delivery Limitations and Regulatory Complexity
- Despite promising clinical data, efficient and safe delivery of RNA molecules to cardiac tissues remains a critical challenge. Unsuch as hepatic targeting (e.g., GalNAc for liver), specific delivery to the heart is more complex, limiting the therapeutic efficacy of some RNA agents
- For instance, while lipid nanoparticles have enabled success in mRNA vaccines and hepatic RNA therapies, their application in cardiac tissue remains underdeveloped, requiring novel vector designs and delivery technologies
- In addition, the evolving and rigorous regulatory environment surrounding RNA-based therapeutics can delay product approvals. Given their novelty and complexity, these therapies must meet stringent standards for long-term safety, immunogenicity, and manufacturing scalability
- The relatively high cost of RNA therapies, especially those using advanced delivery systems or targeting rare cardiac conditions, can also hinder widespread adoption particularly in lower-income regions or healthcare systems with limited reimbursement structures
- Overcoming these challenges through targeted delivery innovations, strategic clinical collaborations, and harmonized regulatory frameworks will be critical for unlocking the full commercial and therapeutic potential of RNA-based treatments in cardiology
RNA-Based Cardiac Therapeutics Market Scope
The market is segmented on the basis of RNA modality, disease indication, developmental stage, and end user.
- By RNA Modality
On the basis of RNA modality, the RNA-based cardiac therapeutics market is segmented into mRNA therapeutics, RNA interference (RNAi), Antisense oligonucleotides (ASOs), MicroRNA (miRNA), Long non-coding RNA (lncRNA), Aptamers, and Small activating RNA (saRNA). The Antisense oligonucleotides (ASOs) segment dominated the market with the largest revenue share of 40% in 2024, owing to its relatively mature development pipeline and demonstrated efficacy in targeting cardiovascular genes such as ApoC-III, ANGPTL3, and Lp(a). ASOs are known for their versatility, high specificity, and favorable safety profile, making them suitable for chronic cardiac conditions such as hypercholesterolemia and heart failure. The segment is strongly supported by ongoing late-stage clinical trials and strategic partnerships from companies such as Ionis Pharmaceuticals and Novartis.
The mRNA therapeutics segment is anticipated to witness the fastest growth rate from 2025 to 2032, driven by innovations in cardiac regeneration and protein expression. Companies are actively exploring mRNA’s potential to stimulate angiogenesis and myocardial repair in ischemic heart disease and heart failure. The success of mRNA technology in vaccines has further accelerated investment and interest in this platform for cardiac applications.
- By Disease Indication
On the basis of disease indication, the RNA-based cardiac therapeutics market is segmented into heart failure, hypercholesterolemia, arrhythmias, ischemic heart disease, cardiomyopathies, and hypertension-related cardiac disorders. The Hypercholesterolemia segment held the largest revenue share in 2024, supported by the success and commercialization of RNA therapies such as inclisiran and the robust pipeline of ASOs and siRNAs targeting lipid disorders. These therapies offer long-acting cholesterol reduction through PCSK9, ApoC-III, and ANGPTL3 modulation, and have gained rapid clinical and regulatory traction in major markets.
The Heart failure segment is expected to witness the fastest CAGR from 2025 to 2032, attributed to the increasing burden of chronic heart conditions and the emergence of RNA-based therapies aimed at modifying gene expression linked to cardiac remodeling and fibrosis. miRNA-targeting agents, particularly those addressing post-infarction damage and myocardial hypertrophy, are rapidly advancing through the clinical pipeline.
- By Developmental Stage
On the basis of developmental stage, the RNA-based cardiac therapeutics market is segmented into discovery / preclinical, phase I, phase II, phase III, and approved / commercialized. The Discovery / Preclinical segment accounted for the largest share in 2024 due to the early-stage nature of many cardiac-focused RNA therapeutics, particularly in emerging modalities such as miRNA, lncRNA, and saRNA. Academic institutions and biotech startups are actively involved in exploratory research targeting a range of cardiovascular genes.
The Phase II segment is projected to grow at the fastest pace from 2025 to 2032, driven by the growing number of mid-stage clinical trials assessing RNA-based drugs for efficacy in lipid modulation, heart failure, and cardiomyopathies. Multiple promising candidates such as CDR132L and pelacarsen are showing positive interim results, signaling an imminent shift toward late-stage development and regulatory submission.
- By End User
On the basis of end user, the RNA-based cardiac therapeutics market is segmented into hospitals & clinics, research & academic institutions, and pharmaceutical & biotechnology companies. The Pharmaceutical & Biotechnology Companies segment dominated the market in 2024, as these stakeholders lead the development, clinical trials, and commercialization of RNA-based cardiac therapeutics. Strategic collaborations, licensing agreements, and funding for RNA-based R&D are concentrated in this segment, especially among key players such as Alnylam, Ionis, and Moderna.
The Hospitals & Clinics segment is projected to grow at the highest CAGR from 2025 to 2032, reflecting the anticipated commercialization of RNA therapies and their adoption in clinical cardiology practice. With increasing physician awareness and supportive reimbursement frameworks, these institutions are expected to play a central role in therapy delivery, particularly for patients with high cardiovascular risk.
RNA-Based Cardiac Therapeutics Market Regional Analysis
- North America dominated the RNA-based cardiac therapeutics market with the largest revenue share of 43.3% in 2024, characterized by advanced healthcare infrastructure, strong R&D funding, early regulatory approvals, and the presence of leading biotech companies pioneering RNA innovation, with the U.S. seeing rapid clinical advancements in RNA drugs targeting heart failure, hyperlipidemia, and arrhythmias
- The region benefits from a well-established biotechnology ecosystem, robust funding from both private and public sectors, and favorable regulatory pathways supporting RNA drug approvals and clinical trials
- This leadership position is further reinforced by the presence of major RNA-focused pharmaceutical companies, increasing patient awareness of gene-targeted treatments, and a healthcare infrastructure that supports the rapid integration of precision therapies into cardiac care
U.S. RNA-Based Cardiac Therapeutics Market Insight
The U.S. RNA-based cardiac therapeutics market captured the largest revenue share of 79.6% in 2024 within North America, driven by the country's advanced biopharmaceutical infrastructure and high prevalence of cardiovascular disorders. Substantial funding for RNA therapeutics, supportive FDA pathways, and an active clinical trial landscape continue to boost innovation. Collaborations between academia and biotech firms, combined with a growing demand for precision medicine, further accelerate the development and commercialization of RNA-based therapies targeting cardiac conditions.
Europe RNA-Based Cardiac Therapeutics Market Insight
The Europe RNA-based cardiac therapeutics market is projected to expand at a robust CAGR throughout the forecast period, supported by rising cardiovascular disease burden and strong government backing for genomic medicine. The region's regulatory agencies are increasingly facilitating RNA therapeutic approvals, while healthcare systems are integrating advanced biologics for better patient outcomes. Europe’s emphasis on translational research and personalized treatment strategies is fostering the growth of RNA-based innovations in the cardiac care sector.
U.K. RNA-Based Cardiac Therapeutics Market Insight
The U.K. RNA-based cardiac therapeutics market is anticipated to grow at a noteworthy CAGR during the forecast period, fueled by the country's proactive stance on genomics research and public-private partnerships. Strategic funding from the NHS and initiatives such as Genomics England are supporting the development of RNA-based therapies. The rising incidence of cardiovascular conditions and the need for innovative, less-invasive treatments are encouraging rapid adoption and investment in RNA therapeutics.
Germany RNA-Based Cardiac Therapeutics Market Insight
The Germany RNA-based cardiac therapeutics market is expected to expand at a considerable CAGR during the forecast period, driven by its strong biotech ecosystem and government-backed R&D efforts. Germany’s emphasis on personalized medicine and growing interest in RNA modalities such as antisense oligonucleotides and siRNA for cardiac disease applications are advancing clinical trials and pipeline development. The country’s robust healthcare infrastructure ensures efficient translation of RNA-based innovations into practice.
Asia-Pacific RNA-Based Cardiac Therapeutics Market Insight
The Asia-Pacific RNA-based cardiac therapeutics market is poised to grow at the fastest CAGR of 25.3% from 2025 to 2032, owing to rapid healthcare modernization, rising cardiovascular disease prevalence, and expanding genomic research across countries such as China, India, and Japan. Governments in the region are increasingly investing in RNA-based drug platforms and personalized care models. The expanding clinical trial ecosystem and cost-effective manufacturing further position Asia-Pacific as a key growth hub for RNA therapeutics in cardiology.
Japan RNA-Based Cardiac Therapeutics Market Insight
The Japan RNA-based cardiac therapeutics market is gaining momentum due to the nation’s technological advancement, aging population, and strong governmental focus on regenerative and precision medicine. Japanese pharma and academic institutions are actively exploring RNA-based treatments for heart failure and arrhythmias, aligning with the country’s broader push toward biotech innovation. Regulatory support and local partnerships continue to foster rapid development and commercialization.
India RNA-Based Cardiac Therapeutics Market Insight
The India RNA-based cardiac therapeutics market accounted for the largest market revenue share in Asia-Pacific in 2024, supported by the country’s rising cardiac disease burden, growing biotechnology sector, and improving access to advanced healthcare. India's government-led genomic initiatives, such as INDIGEN, and increasing clinical trial participation are driving RNA therapeutic development. The availability of skilled researchers and a cost-effective R&D environment position India as a strategic market for RNA-based cardiac innovation.
RNA-Based Cardiac Therapeutics Market Share
The RNA-Based Cardiac Therapeutics industry is primarily led by well-established companies, including:
- Alnylam Pharmaceuticals, Inc. (U.S.)
- Silence Therapeutics plc (U.K.)
- Moderna, Inc. (U.S.)
- CureVac N.V. (Germany)
- BioNTech SE (Germany)
- Arcturus Therapeutics Holdings Inc. (U.S.)
- Arrowhead Pharmaceuticals, Inc. (U.S.)
- Ionis Pharmaceuticals, Inc. (U.S.)
- Sarepta Therapeutics, Inc. (U.S.)
- eTheRNA immunotherapies NV (Belgium)
- TriLink BioTechnologies, LLC (U.S.)
- Lexogen GmbH (Austria)
- AstraZeneca (U.K.)
- Pfizer Inc. (U.S.)
- Sanofi (France)
- Genentech, Inc. (U.S.)
- Ribo Therapeutics, Inc. (U.S.)
- Dicerna Pharmaceuticals, Inc. (U.S.)
- Genevant Sciences Corporation (Canada)
What are the Recent Developments in Global RNA-Based Cardiac Therapeutics Market?
- In May 2024, Moderna, Inc. announced preclinical results of its investigational mRNA therapy targeting myocardial ischemia. The therapy demonstrated significant potential in promoting angiogenesis and improving cardiac function in animal models, signaling a breakthrough in mRNA-based treatment for ischemic heart disease. This development underlines Moderna's continued efforts in expanding its mRNA platform into cardiovascular therapeutics beyond vaccines
- In March 2024, Silence Therapeutics partnered with a major pharmaceutical firm to co-develop RNA interference (RNAi) therapeutics for treating genetic forms of hypertrophic cardiomyopathy. The partnership aims to leverage Silence’s GalNAc-siRNA technology to precisely target hepatic gene expression linked to cardiac conditions, marking a pivotal step in the precision treatment of inherited heart diseases
- In February 2024, AstraZeneca and Ionis Pharmaceuticals advanced their antisense oligonucleotide (ASO) candidate into Phase II trials for treating heart failure with preserved ejection fraction (HFpEF). The candidate showed promise in modulating key proteins involved in cardiac remodeling. This reflects a growing interest in ASO-based strategies for complex and underserved cardiovascular conditions
- In January 2024, BioNTech SE revealed early-stage research into microRNA-based therapies for arrhythmias, including atrial fibrillation. The program, still in discovery phase, focuses on targeting miRNAs that regulate electrical signaling in cardiac tissues. This signals a future shift toward RNA therapeutics in electrophysiology and rhythm management
- In December 2023, Alnylam Pharmaceuticals secured FDA Fast Track designation for its investigational RNAi therapeutic targeting lipoprotein(a), a significant risk factor for atherosclerotic cardiovascular disease. The therapy is intended for patients with hypercholesterolemia and demonstrated encouraging early safety and efficacy results. The regulatory milestone represents a significant advance in RNA-based approaches for lipid-driven heart disease
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.