Global Rna Therapeutics Market
Market Size in USD Billion
CAGR :
% 
 USD
7.05 Billion
USD
19.01 Billion
2024
2032
USD
7.05 Billion
USD
19.01 Billion
2024
2032
| 2025 –2032 | |
| USD 7.05 Billion | |
| USD 19.01 Billion | |
|
|
|
|
RNA Therapeutics Market Size
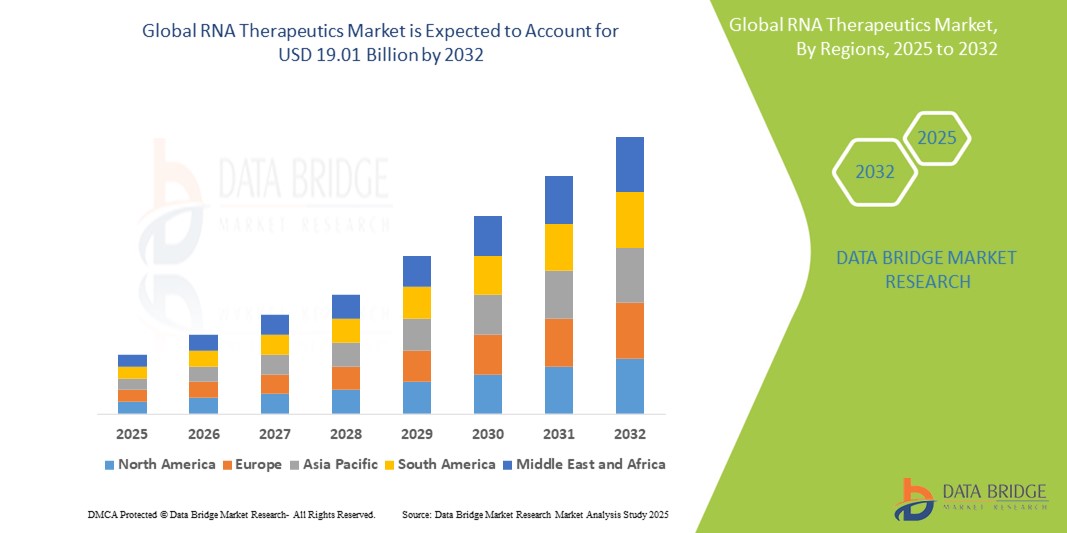
- The global RNA Therapeutics market was valued at USD 7.05 billion in 2024 and is expected to reach USD 19.01 billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 13.20%, primarily driven by the advancements in mRNA technology, increasing prevalence of chronic diseases, and rising investments in personalized medicine
- This growth is driven by factors such as technological advancements in RNA-based drug development, increased funding for biotech research, and the rising demand for targeted and personalized therapies.
RNA Therapeutics Market Analysis
- RNA therapeutics represent a transformative class of medicines that utilize ribonucleic acid (RNA) to treat or prevent diseases by targeting genetic instructions at the molecular level. These therapies include mRNA, siRNA, miRNA, and antisense oligonucleotides used across a wide range of indications, including cancer, genetic disorders, and infectious diseases
- The market’s growth is significantly driven by the increasing prevalence of chronic and genetic diseases, rising demand for precision medicine, and advancements in RNA delivery technologies. The success of mRNA vaccines during the COVID-19 pandemic has also accelerated interest and investment in RNA-based treatments
- North America leads the global RNA therapeutics market, supported by robust biotechnology infrastructure, favorable regulatory frameworks, and strong investment in R&D from both public and private sectors
- For instance, the U.S. has witnessed substantial funding in RNA biotech startups, and major pharmaceutical players are expanding their RNA therapeutic pipelines through collaborations and acquisitions
- Globally, RNA therapeutics are considered one of the most promising frontiers in biomedicine, ranking alongside gene therapy in their potential to revolutionize the treatment landscape for previously untreatable or hard-to-treat conditions
Report Scope and RNA Therapeutics Market Segmentation
|
Attributes |
RNA Therapeutics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
RNA Therapeutics Market Trends
“Expansion of Delivery Technologies and Platform Innovation”
- One prominent trend in the global RNA therapeutics market is the rapid expansion of delivery technologies and innovation in RNA platform
- These Effective delivery remains a critical component for the success of RNA-based therapies, and recent advancements such as lipid nanoparticles (LNPs), exosomes, and polymer-based carriers have significantly improved the stability and targeting of RNA molecules in the body
- For instance, the success of LNPs in delivering mRNA vaccines during the COVID-19 pandemic has paved the way for broader applications of these delivery systems in treating cancer, rare genetic disorders, and infectious diseases
- Platform innovations are also driving the development of next-generation RNA therapies, including self-amplifying RNA (saRNA), circular RNA (circRNA), and RNA editing technologies, which offer enhanced efficacy, reduced dosing frequency, and broader therapeutic potential
- This trend is transforming the RNA therapeutics landscape, accelerating drug development pipelines, and expanding the scope of treatable conditions through RNA-based approaches
RNA Therapeutics Market Dynamics
Driver
“Rising Prevalence of Chronic and Genetic Diseases”
- The increasing incidence of chronic and genetic diseases such as cancer, rare inherited disorders, cardiovascular conditions, and neurodegenerative diseases is a major driver of the global RNA therapeutics market
- These conditions often have limited treatment options or lack effective cures, creating a strong demand for innovative approaches such as RNA-based therapies that target the disease at the molecular or genetic level
- RNA therapeutics, including mRNA, siRNA, and antisense oligonucleotides, offer precise targeting of disease-causing genes, enabling the development of highly specific treatments that can be tailored to individual patients
- The ability of RNA therapeutics to silence, replace, or repair faulty genetic instructions makes them particularly promising for addressing conditions previously considered untreatable
- As healthcare systems worldwide place a greater emphasis on precision medicine and personalized treatment, RNA therapies are gaining traction as a transformative solution across therapeutic areas
For instance,
- In January 2023, the World Health Organization reported that noncommunicable diseases (NCDs), including cancer and cardiovascular disease, are responsible for 74% of all global deaths annually, underscoring the urgent need for novel treatment strategies such as RNA therapeutics
- In September 2022, the U.S. National Institutes of Health announced expanded funding for genetic and rare disease research, supporting the development of RNA-based treatments aimed at targeting the underlying causes of these disorders
- The rising burden of chronic and genetic conditions globally is fueling the demand for RNA therapeutics, driving market growth and accelerating the pace of research and innovation in this space
Opportunity
“Enhancing RNA Therapeutics with Artificial Intelligence and Machine Learning”
- The integration of artificial intelligence (AI) and machine learning (ML) into RNA therapeutics is creating new opportunities for accelerating drug discovery, optimizing RNA sequence design, and predicting therapeutic outcomes with greater accuracy
- AI-powered platforms can analyze large-scale genomic and transcriptomic data to identify novel RNA targets and streamline the development of personalized therapies, significantly reducing time and cost in preclinical and clinical phases
- In addition, ML algorithms can enhance delivery system design by modeling how RNA molecules interact with various carriers, improving stability, targeting, and safety profiles
For instance,
- In February 2024, a study published in Nature Biotechnology highlighted how deep learning models successfully predicted optimal mRNA sequences for protein expression, leading to more efficient vaccine and therapeutic development
- In June 2023, according to the Journal of Translational Medicine, AI-assisted drug discovery platforms helped identify siRNA candidates for previously undruggable targets in neurodegenerative diseases, opening new possibilities for treatment
- The integrating AI across the RNA therapeutic pipeline—from target identification to post-treatment outcome prediction—developers can enhance precision, improve success rates, and expand the range of treatable conditions, making AI a transformative force in the future of RNA-based medicine
Restraint/Challenge
“High Development Costs and Complex Regulatory Pathway”
- The development of RNA therapeutics involves significant financial investment and complex manufacturing processes, posing a major challenge to widespread market adoption and scalability
- Creating stable, effective, and targeted RNA-based drugs requires advanced technologies, high-quality materials, and rigorous testing, which can drive costs into the hundreds of millions of dollars before reaching commercialization
- This regulatory approval for RNA therapeutics is still evolving, as these are relatively new modalities. Navigating diverse and stringent global regulatory frameworks can delay market entry and add uncertainty for developers and investors
For instance,
- In October 2024, a report from the Biotechnology Innovation Organization noted that RNA therapeutics face longer timelines and higher attrition rates during clinical development compared to small-molecule drugs, due to complexities in delivery, immunogenicity, and long-term safety
- In July 2023, according to a publication in Nature Reviews Drug Discovery, the cost of producing lipid nanoparticle (LNP)-encapsulated RNA drugs remains high, especially at commercial scale, limiting accessibility in lower-income regions and creating challenges for broader distribution
- Consequently, high development costs and regulatory challenges continue to limit the pace at which RNA therapeutics can be brought to market, especially for smaller biotech firms and in underserved healthcare markets
RNA Therapeutics Market Scope
The market is segmented on the basis of product type, material, application, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Product Type |
|
|
By Material |
|
|
By Application |
|
|
By Distribution Channel |
|
RNA Therapeutics Market Regional Analysis
“North America is the Dominant Region in the RNA Therapeutics Market”
- North America leads the global RNA therapeutics market, primarily driven by strong biotechnology infrastructure, robust funding for research and development, and a favorable regulatory environment that supports innovation
- The United States accounts for the largest share, owing to the presence of major biotech and pharmaceutical companies, high prevalence of chronic and genetic diseases, and widespread adoption of advanced therapeutic technologies
- The Substantial government and private sector investments in RNA-based research, along with successful commercialization of mRNA vaccines, have further accelerated market expansion in the region
- In addition, active collaboration between academic institutions, biotech firms, and regulatory agencies enhances drug development timelines and supports the early adoption of RNA-based therapies
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- Asia-Pacific is poised to experience the fastest growth in the RNA therapeutics market, supported by increasing investments in biotechnology, rising healthcare expenditures, and a growing focus on precision medicine
- Countries such as China, Japan, South Korea, and India are rapidly advancing in RNA research, propelled by government initiatives and expanding clinical trial activities
- Japan remains a leader in biomedical innovation, with strong regulatory support and high demand for novel therapies to treat aging-related and rare genetic disorders
- In China and India, large patient populations, rising prevalence of chronic diseases, and improvements in healthcare infrastructure are boosting demand for advanced therapies such as RNA-based treatment
- The growing presence of international biotech companies and expanding manufacturing capabilities in the region further contribute to the rapid growth of the RNA therapeutics market in Asia-Pacific
RNA Therapeutics Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Quark Software Inc. (U.S.)
- Alnylam Pharmaceuticals, Inc. (U.S.)
- Novo Nordisk A/S (Denmark)
- Arbutus Biopharma (U.S.)
- Benitec Biopharma Inc. (U.S,)
- Sanofi (France)
- Ionis Pharmaceuticals (U.S.)
- Silence Therapeutics (U.K.)
- Sirnaomics (U.S.)
- CureVac SE (Germany)
- BioNTech SE (Germany)
- Arrowhead Pharmaceuticals, Inc. (U.S.)
- Adhera Health, Inc. (U.S.)
- Moderna, Inc. (U.S.)
- Novartis AG (Switzerland)
- Gilead Sciences, Inc. (U.S.)
- Arcturus Therapeutics, Inc. (U.S.)
- ProQR Therapeutics (Netherlands)
- Phio Pharmaceuticals (U.S.)
- GreenLight Biosciences, Inc. (U.S.)
Latest Developments in Global RNA Therapeutics Market
- In October 2024, Sarepta Therapeutics faced a significant setback when European regulators paused all clinical trials of its gene therapy, Elevidys, following the death of a U.S. teenager treated with the drug. Elevidys targets Duchenne muscular dystrophy and had been administered to over 800 patients prior to this incident
- In July 2024, GSK acquired CureVac's stakes in their collaborative influenza and COVID-19 vaccine development, including an upfront payment of 400 million euros and potential milestone payments up to 1.05 billion euros. This move aims to strengthen GSK's mRNA technology capabilities
- In March 2024, Eli Lilly's experimental drug, lepodisiran, demonstrated promising results by reducing lipoprotein(a) levels by 94% for six months with a single shot in a Phase 2 trial. Elevated lipoprotein(a) is a significant risk factor for heart attacks and strokes
- In October 2023, Novo Nordisk received FDA approval for Rivfloza, an RNA interference (RNAi) therapy designed to treat a rare kidney-affecting genetic disorder. This therapy utilizes RNAi technology to lower urinary oxalate levels by silencing genes that contribute to the disease
- In July 2023: Alnylam Pharmaceuticals entered into a strategic agreement with Roche to develop and commercialize zilebesiran, an investigational RNAi therapeutic in Phase 2 development for the treatment of hypertension. This collaboration aims to advance RNAi-based treatments for cardiovascular conditions
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.