Global Schnitzler Syndrome Disease Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
143.10 Million
USD
195.84 Million
2024
2032
USD
143.10 Million
USD
195.84 Million
2024
2032
| 2025 –2032 | |
| USD 143.10 Million | |
| USD 195.84 Million | |
|
|
|
|
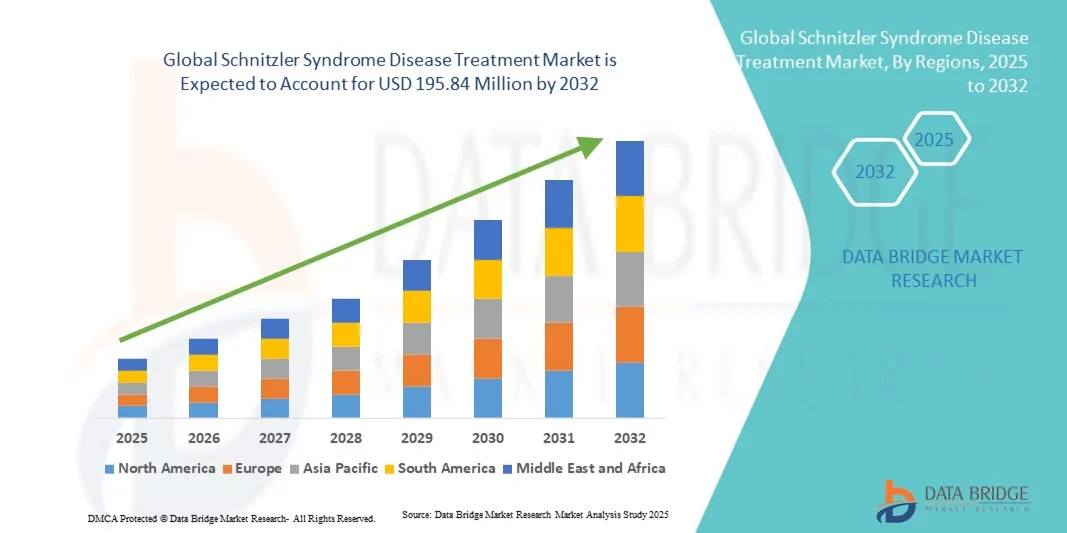
Schnitzler Syndrome Disease Treatment Market Size
- The global Schnitzler Syndrome disease treatment market size was valued at USD 143.10 million in 2024 and is expected to reach USD 195.84 million by 2032, at a CAGR of 4.00% during the forecast period
- The market growth is primarily driven by increasing clinical awareness, advancements in diagnostic techniques, and the growing availability of targeted therapies particularly interleukin-1 (IL-1) inhibitors such as anakinra and canakinumab, which have demonstrated significant efficacy in managing the syndrome’s chronic inflammatory symptoms
- Furthermore, rising investments in rare disease research, improved patient registries, and enhanced access to biologic treatments are propelling the market forward. These combined factors are fostering early diagnosis, expanding treatment adoption, and ultimately strengthening the growth trajectory of the global Schnitzler Syndrome disease treatment market
Schnitzler Syndrome Disease Treatment Market Analysis
- Schnitzler Syndrome disease treatment focuses on addressing this rare autoinflammatory condition characterized by recurrent fevers, urticarial rashes, bone pain, and systemic inflammation. Therapeutic management primarily aims to alleviate symptoms, control inflammation, and improve quality of life using targeted immunomodulatory and anti-inflammatory agents
- The market growth is driven by increasing clinical awareness, expanding access to advanced diagnostics, and the growing adoption of biologic and immunosuppressive therapies to manage chronic inflammatory symptoms. Rising research collaborations and orphan drug approvals are further accelerating treatment advancements
- North America dominated the Schnitzler Syndrome disease treatment market with the largest revenue share of 42.4% in 2024, supported by strong healthcare infrastructure, growing R&D investments in rare diseases, and wider clinical availability of IL-1 inhibitors such as anakinra and canakinumab
- Asia-Pacific is expected to be the fastest-growing region during the forecast period, driven by improving healthcare infrastructure, growing awareness of rare disorders, and increasing adoption of biologic treatments in countries such as Japan, China, and South Korea
- The Corticosteroids segment dominated the market with a 43.7% share in 2024, owing to their established use as first-line therapy for managing inflammatory flares and their accessibility compared to biologic or immunosuppressive alternatives
Report Scope and Schnitzler Syndrome Disease Treatment Market Segmentation
|
Attributes |
Schnitzler Syndrome Disease Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Schnitzler Syndrome Disease Treatment Market Trends
Advancements in Targeted Biologic Therapies and Cytokine Inhibition
- A significant and accelerating trend in the global Schnitzler Syndrome disease treatment market is the growing adoption of biologic therapies targeting cytokine pathways, particularly interleukin-1 (IL-1) and interleukin-6 (IL-6), which are central to the disease’s inflammatory process
- For instance, anakinra (Kineret) and canakinumab (Ilaris) have shown remarkable clinical success in reducing systemic inflammation and improving patient outcomes by specifically blocking IL-1 activity. Similarly, newer IL-6 inhibitors are being evaluated for refractory cases, expanding therapeutic options
- The integration of biologic drugs into treatment protocols enables more precise disease control, minimizing the reliance on broad-spectrum anti-inflammatory agents and reducing long-term corticosteroid dependence. This advancement is significantly improving patient quality of life and long-term prognosis
- Furthermore, ongoing clinical trials and real-world evidence studies are enhancing understanding of long-term efficacy, safety profiles, and combination therapy strategies involving biologics and immunosuppressants for sustained remission
- The increasing use of precision medicine approaches and biomarker-driven patient selection is paving the way for more individualized treatment regimens, optimizing therapeutic effectiveness in complex autoinflammatory disorders
- This trend toward targeted, mechanism-based treatments is fundamentally reshaping clinical management, encouraging pharmaceutical innovation, and expanding the therapeutic landscape for rare systemic inflammatory conditions such as Schnitzler Syndrome
Schnitzler Syndrome Disease Treatment Market Dynamics
Driver
Growing Diagnosis Rates and Advancements in Rare Disease Therapeutics
- The rising awareness and clinical recognition of Schnitzler Syndrome among healthcare professionals, coupled with improved access to genetic and immunological diagnostic tools, are significantly driving treatment demand and early disease management
- For instance, in March 2024, Novartis AG advanced clinical research on canakinumab for chronic autoinflammatory syndromes, aiming to enhance therapeutic outcomes in rare conditions including Schnitzler Syndrome. Such R&D initiatives are expected to strengthen the global treatment pipeline
- As more specialized centers adopt next-generation sequencing and immunopathological testing, the detection of rare autoinflammatory disorders is improving, leading to earlier therapeutic intervention and better patient outcomes
- Furthermore, strong government support and orphan drug policies across major markets are accelerating drug development, clinical access, and affordability for rare inflammatory diseases
- The growing use of biologics, along with increasing collaborations between research institutions and pharmaceutical companies, continues to expand the available treatment portfolio and clinical understanding of the disease
- The expanding patient registries, real-world data collection, and continuous innovation in immunomodulatory drugs are collectively propelling the growth of the Schnitzler Syndrome disease treatment market globally
Restraint/Challenge
High Cost of Biologic Therapies and Limited Clinical Awareness
- The elevated cost associated with biologic and targeted immunotherapies poses a major barrier to widespread treatment adoption, especially in low- and middle-income regions where healthcare reimbursement remains limited
- For instance, patients receiving IL-1 inhibitors such as anakinra or canakinumab often face high annual treatment expenses, making affordability and long-term adherence significant challenges
- Limited physician familiarity with Schnitzler Syndrome due to its rarity frequently results in delayed or incorrect diagnosis, preventing timely initiation of effective therapies and increasing disease burden
- Furthermore, the scarcity of large-scale clinical trials and a limited patient pool hinder the development of standardized treatment guidelines and regulatory approvals for emerging biologics
- The dependence on specialist centers for diagnosis and therapy administration further restricts accessibility, particularly in regions lacking rheumatology or immunology expertise
- Addressing these challenges through improved physician education, wider healthcare coverage for orphan drugs, and increased investment in clinical research will be essential for the sustained growth of this market
Schnitzler Syndrome Disease Treatment Market Scope
The market is segmented on the basis of symptoms and treatment.
- By Symptoms
On the basis of symptoms, the Schnitzler Syndrome disease treatment market is segmented into recurrent fevers, joint pain and inflammation, organomegaly, bone pain, blood abnormalities, muscle aches, fatigue, weight loss, and others. The Recurrent Fevers segment dominated the market with the largest market revenue share in 2024, driven by the symptom’s frequency and its role as a primary clinical trigger prompting specialist referral and treatment initiation. Recurrent fevers often lead to urgent medical evaluation, increasing short-term utilization of rapid-acting therapies and diagnostic resources. Physicians commonly start fast-onset agents such as corticosteroids to control febrile flares, which elevates prescription volumes and near-term market spend. Clinical trials and real-world studies frequently measure fever reduction as a core endpoint, focusing R&D and payer interest on therapies that rapidly suppress fevers. Because fevers correlate with systemic inflammatory burden, patients with frequent flares require ongoing monitoring and maintenance therapy, supporting sustained market demand. Lastly, clear symptomatic improvement in fever control increases patient retention on advanced therapies, reinforcing the revenue weight of this subsegment.
The Blood Abnormalities segment is expected to witness the fastest growth rate from 2025 to 2032, fueled by improved detection of monoclonal gammopathy and tighter links between hematologic findings and disease management. Greater use of serum protein electrophoresis and immunofixation in diagnostic workflows is expanding the diagnosed patient pool with relevant blood abnormalities. Recognition of gammopathy’s clinical relevance is driving referrals to hematology, opening pathways for combined immunologic-hematologic treatment approaches and higher-value care. Interest in B-cell directed strategies and off-label applications of hematologic agents in Schnitzler patients is prompting new clinical investigations and adoption. Payer recognition of hematologic monitoring and combined treatment regimens is increasing reimbursement for diagnostics and adjunct therapies, supporting revenue growth. Collaborative research between immunology and hematology centers is accelerating the development of protocols addressing blood abnormalities, further boosting uptake in this subsegment.
- By Treatment
On the basis of treatment, the Schnitzler Syndrome disease treatment market is segmented into Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), corticosteroids, immunosuppressive agents, colchicine, dapsone, thalidomide, rituximab, and others. The Corticosteroids segment dominated the market with the largest market revenue share of 43.7% in 2024, reflecting their widespread use as rapid, accessible anti-inflammatory treatment for acute flares. Corticosteroids deliver immediate symptomatic relief for fever, rash, and pain, driving frequent use at disease onset and during exacerbations. Their low acquisition cost and broad availability across inpatient and outpatient settings sustain high prescription volumes globally. Clinicians often use steroids as bridging therapy while initiating disease-modifying agents, maintaining steady demand despite known long-term adverse effects. In regions with limited access to biologics, corticosteroids remain the principal accessible option, further reinforcing their revenue dominance. Guideline recommendations for acute symptom control and entrenched physician familiarity also contribute to the corticosteroid segment’s leading share.
The Immunosuppressive Agents segment is expected to witness the fastest CAGR from 2025 to 2032, driven by growing adoption of targeted immunomodulatory and biologic strategies in refractory and steroid-sparing care. Increasing clinical evidence for steroid-sparing benefits and durable responses with immunosuppressants encourages earlier specialist adoption. Regulatory incentives and improving reimbursement frameworks for rare-disease therapies are making higher-cost immunosuppressive regimens more accessible to patients. Ongoing trials and real-world studies demonstrating improved long-term outcomes with immunomodulators are expanding prescriber confidence and use. Multidisciplinary treatment models that combine rheumatology, immunology, and hematology favor complex immunosuppressive protocols, raising specialist-driven prescriptions. Advances in biomarker-guided therapy selection are enabling more precise patient matching to immunosuppressive options, accelerating uptake and revenue growth for this subsegment.
Schnitzler Syndrome Disease Treatment Market Regional Analysis
- North America dominated the Schnitzler Syndrome disease treatment market with the largest revenue share of 42.4% in 2024, supported by strong healthcare infrastructure, growing R&D investments in rare diseases, and wider clinical availability of IL-1 inhibitors such as anakinra and canakinumab
- Patients and healthcare providers in the region increasingly adopt innovative biologic treatments such as IL-1 inhibitors due to their proven efficacy in controlling chronic inflammation and improving patient quality of life
- This strong market presence is further supported by robust R&D investment, active rare disease programs, and favorable reimbursement policies for orphan drugs, establishing North America as the leading region for Schnitzler Syndrome disease treatment adoption and development
U.S. Schnitzler Syndrome Disease Treatment Market Insight
The U.S. Schnitzler Syndrome disease treatment market captured the largest revenue share of 82% in 2024 within North America, driven by high disease awareness, early diagnosis rates, and the strong presence of specialized immunology and rheumatology centers. The availability of advanced biologic treatments such as IL-1 inhibitors and ongoing clinical trials for novel monoclonal antibodies are propelling market growth. Supportive regulatory policies for orphan diseases and active participation by major pharmaceutical companies further enhance treatment accessibility. Moreover, robust healthcare spending and increasing adoption of precision medicine approaches are solidifying the U.S. as a global leader in Schnitzler Syndrome treatment innovation.
Europe Schnitzler Syndrome Disease Treatment Market Insight
The Europe Schnitzler Syndrome disease treatment market is projected to expand at a significant CAGR during the forecast period, primarily due to the strong network of rare disease research programs and supportive government funding for orphan drug development. Rising collaborations between academic institutions and biopharmaceutical companies are accelerating advancements in biologic and immunomodulatory therapies. Patients in Europe benefit from well-established diagnostic frameworks and access to advanced care centers specializing in autoinflammatory disorders. In addition, stringent healthcare regulations emphasizing efficacy and patient safety continue to shape therapeutic adoption across the region.
U.K. Schnitzler Syndrome Disease Treatment Market Insight
The U.K. Schnitzler Syndrome disease treatment market is expected to grow steadily, supported by improved clinical recognition and access to specialist treatment centers under the National Health Service (NHS). Government-backed initiatives promoting rare disease research and early diagnostic programs are driving greater awareness and treatment adoption. The increasing availability of biologics such as anakinra and canakinumab, along with ongoing research into targeted immune therapies, is contributing to the expansion of the market. Furthermore, the U.K.’s emphasis on patient-centric care and digital health integration enhances treatment monitoring and management for chronic autoinflammatory conditions.
Germany Schnitzler Syndrome Disease Treatment Market Insight
The Germany Schnitzler Syndrome disease treatment market is poised for considerable growth, fueled by advanced medical infrastructure and a strong focus on immunology and rare disease research. The country’s comprehensive healthcare system facilitates access to biologic treatments and diagnostic services, driving higher treatment uptake. Germany’s active participation in European clinical trials for IL-1 blockade therapies is further expanding therapeutic options for patients. In addition, favorable reimbursement structures and the presence of leading pharmaceutical companies engaged in rare disease innovation underpin market expansion.
Asia-Pacific Schnitzler Syndrome Disease Treatment Market Insight
The Asia-Pacific Schnitzler Syndrome disease treatment market is expected to grow at the fastest CAGR from 2025 to 2032, driven by rising awareness of rare autoinflammatory disorders and expanding healthcare infrastructure in countries such as Japan, China, and India. Increased investments in diagnostic capabilities, combined with the introduction of biologics in emerging markets, are transforming the regional treatment landscape. Government initiatives promoting rare disease registries and access to innovative therapies are further stimulating market growth. Moreover, growing participation in international clinical research and partnerships with Western pharmaceutical firms are enhancing the region’s role in Schnitzler Syndrome management.
Japan Schnitzler Syndrome Disease Treatment Market Insight
The Japan Schnitzler Syndrome disease treatment market is gaining traction due to the nation’s advanced healthcare system, growing focus on precision medicine, and the rising prevalence of rare disease awareness programs. Japan’s strong regulatory support for orphan drugs and ongoing clinical evaluations of biologic therapies such as canakinumab are fostering significant market expansion. The integration of advanced genetic testing and patient registries aids in early detection and effective treatment planning. In addition, collaborations between research institutes and global biotech firms are strengthening Japan’s position in the rare disease therapeutics space.
India Schnitzler Syndrome Disease Treatment Market Insight
The India Schnitzler Syndrome disease treatment market accounted for the largest market share within Asia-Pacific in 2024, attributed to expanding diagnostic capabilities, improved access to specialist care, and increasing adoption of biologic treatments. Government initiatives to enhance rare disease management, coupled with growing participation of domestic pharmaceutical manufacturers, are boosting availability and affordability of therapies. Awareness campaigns and patient advocacy groups are helping identify cases earlier, thereby improving treatment outcomes. Furthermore, ongoing clinical collaborations and the development of biosimilar biologics are expected to make India a key emerging hub for Schnitzler Syndrome treatment advancement.
Schnitzler Syndrome Disease Treatment Market Share
The Schnitzler Syndrome Disease Treatment industry is primarily led by well-established companies, including:
- Novartis AG (Switzerland)
- Swedish Orphan Biovitrum AB (Sweden)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Sanofi. (France)
- Regeneron Pharmaceuticals, Inc. (U.S.)
- AbbVie Inc. (U.S.)
- Amgen Inc. (U.S.)
- Pfizer Inc. (U.S.)
- AstraZeneca plc (U.K.)
- Takeda Pharmaceutical Company Limited (Japan)
- Eli Lilly and Company (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- Biogen Inc. (U.S.)
- UCB S.A. (Belgium)
- Astellas Pharma Inc. (Japan)
- Bayer AG (Germany)
- Merck & Co., Inc. (U.S.)
- GSK plc (U.K.)
- Vertex Pharmaceuticals Incorporated (U.S.)
What are the Recent Developments in Global Schnitzler Syndrome Disease Treatment Market?
- In April 2025, an investigator-initiated, multi-centre, single-arm, open-label study of Canakinumab in Japanese patients with Schnitzler Syndrome reported that 3 of 5 patients achieved complete response by Day 7, supporting the drug’s potential utility in a new regional population
- In January 2025, a case report described the therapeutic use of Bortezomib a proteasome inhibitor typically used in multiple myeloma in a patient with Schnitzler Syndrome, suggesting a novel off-label collaboration between hematology/oncology and autoinflammatory disease specialists
- In September 2023, a peer-reviewed article titled “Schnitzler syndrome An underdiagnosed adult-onset autoinflammatory disease” highlighted the expanding therapeutic role of IL-1 blockers (e.g., anakinra) and urged increased global diagnostic and treatment collaborations
- In March 2023, a long-term observational study titled “Canakinumab leads to rapid reduction of neutrophilic inflammation and long-lasting response in Schnitzler syndrome” reported sustained remission over 10 years in a patient treated with Canakinumab. This underscores collaboration between academic centres and industry in real-world evidence generation
- In July 2021, clinical evidence reported a case of SchS with extremely elevated IL-6 levels where standard therapies failed; yet, after switching to an IL-1 receptor antagonist (Anakinra), significant improvement occurred illustrating collaboration in treatment strategy beyond initial targets
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.