Global Scleroderma Drugs Market
Market Size in USD Billion
CAGR :
% 
 USD
1.48 Billion
USD
2.97 Billion
2024
2032
USD
1.48 Billion
USD
2.97 Billion
2024
2032
| 2025 –2032 | |
| USD 1.48 Billion | |
| USD 2.97 Billion | |
|
|
|
|
Scleroderma Drugs Market Analysis
The scleroderma drugs market is experiencing steady growth driven by increasing awareness of the disease, advancements in treatment options, and the rising prevalence of autoimmune disorders. Scleroderma, a chronic condition, has led to a greater focus on developing effective therapies. Pharmaceutical companies are investing in research to develop targeted treatments that address the underlying causes of scleroderma, including immune modulation and anti-fibrotic therapies.
Currently, drugs such as immunosuppressants, biologics, and vasodilators are used to manage the symptoms and slow disease progression. Additionally, the approval of newer therapies, such as tocilizumab for systemic sclerosis, has expanded the range of treatment options available to patients. The growing emphasis on personalized medicine, tailored to individual patient needs, also contributes to market growth by offering more precise and effective treatment regimens.
Moreover, the increasing number of clinical trials and a better understanding of the disease's molecular mechanisms are expected to accelerate the development of novel treatments. Challenges such as the high cost of advanced therapies and limited treatment options for certain types of scleroderma remain, but the ongoing progress in drug development offers optimism for improving patient outcomes and driving market expansion in the coming years.
Scleroderma Drugs Market Size
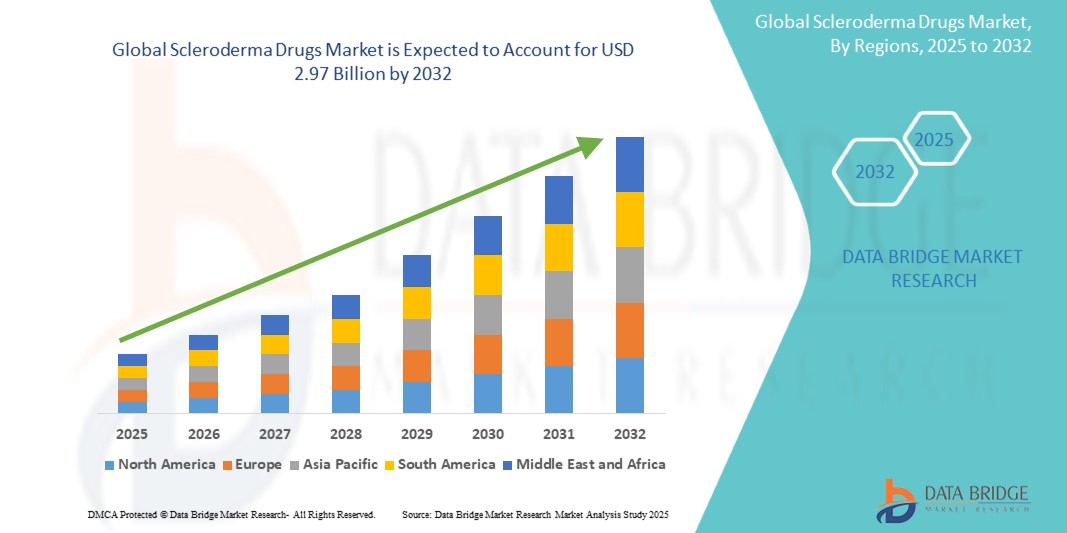
The global scleroderma drugs market size was valued at USD 1.48 billion in 2024 and is projected to reach USD 2.97 billion by 2032, with a CAGR of 9.20% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Scleroderma Drugs Market Trends
“Developing targeted therapies”
The scleroderma drugs market is thriving primarily due to the growing emphasis on developing targeted therapies that address the significant unmet needs of patients with this rare and complex autoimmune disorder. As awareness of scleroderma continues to rise, there has been an upsurge in research and clinical trials focused on identifying more effective treatments. Recent advancements in understanding the disease’s underlying mechanisms have led to the development of innovative therapies, including biologics such as tocilizumab, which specifically target immune dysfunction and fibrosis. The increasing shift toward personalized medicine is also driving market growth, as treatments are becoming more tailored to individual patient profiles, enhancing their efficacy. Additionally, the expanding pipeline of new drugs and the growing recognition of the profound impact of scleroderma on patients' quality of life further contribute to market expansion. Collaborations between pharmaceutical companies and research institutions are accelerating the development of novel therapies, strengthening the market’s potential.
Report Scope and Scleroderma Drugs Market Segmentation
|
Attributes |
Scleroderma Drugs Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Italy, Spain, Austria, Denmark, Sweden, Norway, Rest of Europe in Europe, China, Japan, India, Malaysia, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Kuwait, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Key Market Players |
Akashi RX (U.S.), Bayer AG (Germany), Boehringer Ingelheim International GmbH (Germany), Bristol-Myers Squibb Company (U.S.), Cabaletta Bio, Inc. (U.S.), Certa Therapeutics (Australia), Chugai Pharmaceutical Co., Ltd. (Japan), Corbus Pharmaceuticals Holdings, Inc. (U.S.), Corvus Pharmaceuticals (U.S.), Gesynta Pharma AB (Sweden), GSK plc. (UK), Kyowa Kirin Co., Ltd. (Japan), Kyverna Therapeutics, Inc. (U.S.), Liminal BioSciences Inc. (Canada), Merck & Co., Inc. (U.S.), Pfizer Inc. (U.S.), Sanofi (France), Skye Bioscience (U.S.), United Therapeutics Corporation (U.S.), Zura Bio Ltd. (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Scleroderma Drugs Market Definition
Scleroderma is a rare, chronic autoimmune disease that causes the body’s immune system to attack its own tissues, leading to the thickening and hardening of the skin and connective tissues. This condition can also affect internal organs, such as the lungs, heart, kidneys, and digestive system. The two main types of scleroderma are localized scleroderma, which mainly affects the skin, and systemic sclerosis, which can involve the skin as well as internal organs. Scleroderma drugs are medications used to treat the symptoms and complications of scleroderma, a chronic autoimmune disease that causes the hardening and tightening of the skin and connective tissues. These drugs aim to manage symptoms, slow disease progression, and improve the quality of life for patients.
Scleroderma Drugs Market Dynamics
Drivers
- Increasing Prevalence of Scleroderma
The growing global prevalence of scleroderma, especially systemic sclerosis, significantly drives the demand for targeted treatments. As the number of diagnosed patients rises, there is an increasing need for effective therapies to manage both skin and organ involvement. For instance, the FDA-approved Ofev (nintedanib) has demonstrated efficacy in slowing the progression of pulmonary fibrosis associated with systemic sclerosis, addressing a major complication in these patients. This rising patient population contributes to greater market demand for new drugs, driving pharmaceutical companies to focus on developing innovative therapies. With more patients seeking treatment, the need for drugs that manage symptoms, prevent organ damage, and improve the quality of life is expected to continue growing, fueling market expansion.
- Advancements in Drug Development
Continued advancements in drug development and ongoing clinical trials are key drivers for the growth of the scleroderma drugs market. As researchers deepen their understanding of scleroderma’s underlying mechanisms, more targeted therapies are emerging. For instance, Certa Therapeutics’ FT011, an investigational therapy for systemic sclerosis, has received Fast Track Designation from the FDA. This highlights the growing focus on developing treatments that address the immune system's role in scleroderma. Furthermore, the rising interest in biologics and personalized medicine is propelling the development of drugs that can target specific molecular pathways involved in the disease. As new drugs enter the pipeline, the market is expected to see substantial growth.
Opportunities
- Expansion of Biologic Therapies
The increasing focus on biologic therapies presents a significant opportunity in the scleroderma drugs market. Biologics, which target specific immune system components involved in autoimmune diseases, offer the potential for more effective treatments with fewer side effects. For instance, Ofev (nintedanib) and the investigational biologic, tocilizumab, have shown promising results in treating systemic sclerosis-associated interstitial lung disease (SSc-ILD), highlighting the potential of biologic therapies. As researchers uncover more about the disease's underlying mechanisms, the opportunity to develop new biologic drugs tailored to treat specific aspects of scleroderma is expanding. With personalized medicine gaining traction, biologics offer a unique opportunity to improve outcomes for patients with complex disease manifestations, thus driving market growth.
- Emerging Market Penetration
An important growth opportunity for the scleroderma drugs market lies in expanding access to treatments in emerging markets. As healthcare infrastructure improves in regions such as Asia-Pacific and Latin America, the demand for scleroderma therapies is increasing. For instance, the approval of drugs such as Ofev in these regions provides an opportunity for pharmaceutical companies to tap into a previously underserved market. With the rising awareness of autoimmune diseases and the growing healthcare capacity in these regions, companies can introduce new treatments and expand their market presence. This growing patient pool presents a significant opportunity for pharmaceutical companies to increase sales and contribute to global access to essential therapies.
Restraints/Challenges
- High Cost of Treatment
A major restraint in the scleroderma drugs market is the high cost of treatment, particularly for advanced therapies such as biologics. Drugs such as Ofev (nintedanib) and other novel biologic therapies are often expensive, which can limit accessibility for many patients, especially in low- and middle-income countries. For instance, the annual cost of Ofev can be prohibitively high, making it difficult for patients to afford long-term treatment. This high cost also places a financial burden on healthcare systems and insurance providers, further limiting the widespread adoption of these treatments and the overall market growth.
- Limited Treatment Options for Certain Scleroderma Subtypes
A key challenge in the scleroderma drugs market is the limited treatment options for specific subtypes of the disease. While therapies such as Ofev are effective for systemic sclerosis-associated interstitial lung disease (SSc-ILD), treatment options remain sparse for other manifestations, such as skin involvement or vascular complications. Additionally, scleroderma’s heterogeneous nature means that treatment efficacy can vary widely among patients. This lack of targeted therapies for all forms of scleroderma poses a challenge for comprehensive treatment strategies, making it difficult to address the full spectrum of symptoms and disease progression, thereby hindering the growth of the market.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Scleroderma Drugs Market Scope
The market is segmented on the basis of drug class, indication, and route of administration. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Drug Class
- Immunosuppressors
- Phosphodiesterase 5 inhibitors - PHA
- Endothelin Receptor Antagonists
- Prostacyclin Analogues
- Calcium Channel Blockers
- Analgesics
- Others
Indication
- Systemic Scleroderma
- Morphea
- Linear
- Localized Scleroderma
- Diffuse Systemic Sclerosis
- Limited Cutaneous Systemic Sclerosis Syndrome
Route of Administration
- Oral
- Injectable
- Others
Scleroderma Drugs Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, drug class, indication, and route of administration as referenced above.
The countries covered in the market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Italy, Spain, Austria, Denmark, Sweden, Norway, Rest of Europe in Europe, China, Japan, India, Malaysia, South Korea, Australia, Thailand, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Kuwait, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
The North American region is expected to dominate the scleroderma drugs market. The dominance is primarily driven by the well-established healthcare infrastructure, high healthcare spending, and the presence of leading pharmaceutical companies that are advancing research and drug development for scleroderma. Additionally, the growing awareness of autoimmune diseases, including scleroderma, and the increasing number of FDA-approved therapies for the condition contribute to the market's growth in North America. The availability of advanced treatments such as biologics and immunosuppressive drugs further fuels the demand for scleroderma drugs in this region.
The Asia-Pacific (APAC) region is expected to exhibit the highest growth rate in the scleroderma drugs market. This growth is driven by the improving healthcare infrastructure, rising healthcare awareness, and the increasing prevalence of autoimmune diseases in the region. Countries such as China and India are witnessing advancements in healthcare access, which is enhancing the diagnosis and treatment of rare diseases such as scleroderma. Additionally, the growing demand for innovative therapies, including biologics, is contributing to the market expansion in APAC. As more pharmaceutical companies focus on entering these emerging markets with new scleroderma drugs, the region is anticipated to experience substantial market growth in the coming years.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Scleroderma Drugs Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Scleroderma Drugs Market Leaders Operating in the Market Are:
- Akashi RX (U.S.)
- Bayer AG (Germany)
- Boehringer Ingelheim International GmbH (Germany)
- Bristol-Myers Squibb Company (U.S.)
- Cabaletta Bio, Inc. (U.S.)
- Certa Therapeutics (Australia)
- Chugai Pharmaceutical Co., Ltd. (Japan)
- Corbus Pharmaceuticals Holdings, Inc. (U.S.)
- Corvus Pharmaceuticals (U.S.)
- Gesynta Pharma AB (Sweden)
- GSK plc. (UK)
- Kyowa Kirin Co., Ltd. (Japan)
- Kyverna Therapeutics, Inc. (U.S.)
- Liminal BioSciences Inc. (Canada)
- Merck & Co., Inc. (U.S.)
- Pfizer Inc. (U.S.)
- Sanofi (France)
- Skye Bioscience (U.S.)
- United Therapeutics Corporation (U.S.)
- Zura Bio Ltd. (U.S.)
Latest Developments in Scleroderma Drugs Market
- In November 2024, Corvus Pharmaceuticals, Inc., a clinical-stage biopharmaceutical company, announced new preclinical data demonstrating the potential of soquelitinib, the company’s lead ITK inhibitor program, to prevent lung damage, inflammation, and pulmonary hypertension associated with systemic sclerosis. The preclinical findings, presented at ACR, confirm the drug’s potential for treating immune-mediated fibrotic diseases such as systemic sclerosis.
- In March 2024, Cabaletta Bio, Inc. announced that the U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) to CABA-201, an investigational 4-1BB-containing fully human CD19-CAR T cell therapy, for the treatment of systemic sclerosis (SSc). CABA-201 is being developed as a potential therapy for autoimmune diseases driven by B cells.
- In February 2024, Certa Therapeutics (Certa) announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track Designation to its investigational therapy, FT011, for the treatment of systemic sclerosis (scleroderma), following the earlier Orphan Drug Designation. The Fast-Track Designation was awarded bassed on the results of a previously reported Phase 2 study, which showed that 12 weeks of treatment with FT011 led to a clinically meaningful improvement in 60% of patients receiving the 400mg dose and 20% of patients receiving the 200mg dose, compared to just 10% in the placebo group.
- In October 2023, Kyverna Therapeutics announced that the U.S. Food and Drug Administration (FDA) had cleared its third Investigational New Drug (IND) application for KYV-101. This approval enables Kyverna to launch a Phase 1/2 open-label, multicenter study of KYV-101, an autologous fully human anti-CD19 Chimeric Antigen Receptor (CAR) T cell therapy, for the treatment of diffuse cutaneous systemic sclerosis (scleroderma).
- In February 2023, GSK plc announced that the U.S. Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) to Benlysta (belimumab), a monoclonal antibody that inhibits B-cells, for the potential treatment of systemic sclerosis. GSK intends to begin a Phase II/III trial of belimumab for systemic sclerosis-associated interstitial lung disease (SSc-ILD) in the first half of 2023.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.