Global Sialidosis Market
Market Size in USD Million
CAGR :
% 
 USD
430.89 Million
USD
631.79 Million
2025
2033
USD
430.89 Million
USD
631.79 Million
2025
2033
| 2026 –2033 | |
| USD 430.89 Million | |
| USD 631.79 Million | |
|
|
|
|
Sialidosis Market Size
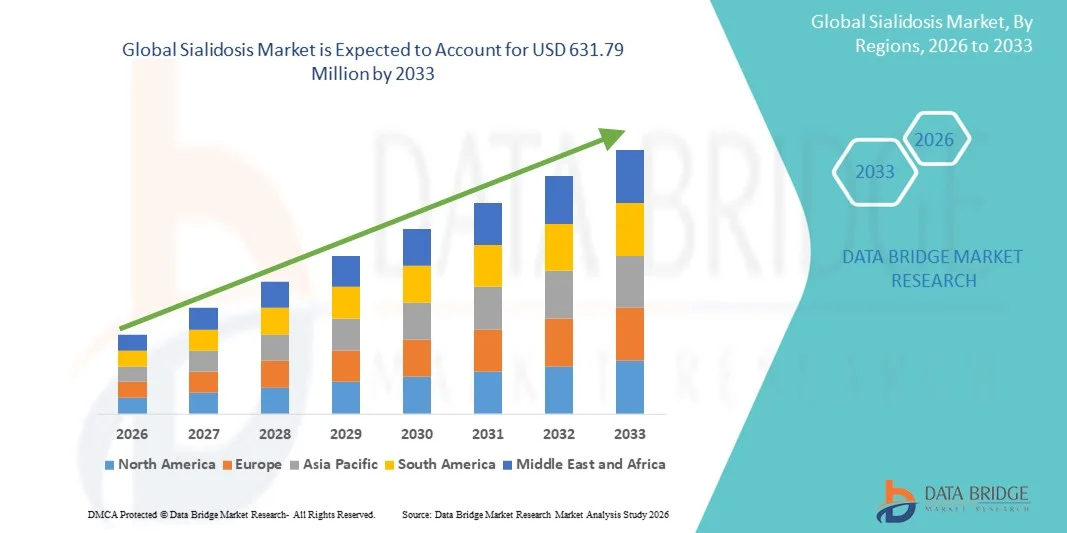
- The global sialidosis market size was valued at USD 430.89 million in 2025 and is expected to reach USD 631.79 million by 2033, at a CAGR of 4.90% during the forecast period
- The market growth is largely fueled by the increasing focus on rare disease diagnosis and advancements in enzyme replacement and gene therapy approaches, which are enhancing clinical outcomes for patients with lysosomal storage disorders such as sialidosis
- Furthermore, rising research funding, growing awareness of early genetic screening, and the expanding pipeline of novel therapeutics are positioning sialidosis treatments as a vital area within the rare disease segment. These converging factors are driving market expansion and accelerating innovation in sialidosis therapy worldwide
Sialidosis Market Analysis
- Sialidosiss, a rare lysosomal storage disorder caused by NEU1 gene mutations, is increasingly gaining attention in both clinical and research settings due to its progressive nature and the potential for targeted therapies, including genetic counseling and supportive treatments
- The escalating focus on sialidosis is primarily fueled by growing awareness of rare genetic disorders, advancements in diagnostic techniques such as blood, urine, and skin biopsy tests, and an expanding pipeline of novel therapeutics aimed at improving patient outcomes
- North America dominated the sialidosis market with the largest revenue share of 41.4% in 2025, driven by advanced healthcare infrastructure, high investment in rare disease research, and early adoption of genetic screening programs, with the U.S. leading in clinical trials and treatment access
- Asia-Pacific is expected to be the fastest growing region in the sialidosis market during the forecast period due to increasing healthcare accessibility, rising awareness of rare diseases, and government initiatives supporting diagnostics and therapy development
- Sialidosis Type I segment dominated the market with a market share of 52.8% in 2025, driven by its relatively milder progression, better diagnostic recognition, and increasing treatment availability compared to Sialidosis Type II
Report Scope and Sialidosis Market Segmentation
|
Attributes |
Sialidosis Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Sialidosis Market Trends
Increasing Focus on Early Diagnosis and Genetic Screening
- A significant and accelerating trend in the global sialidosis market is the growing emphasis on early diagnosis through genetic screening and advanced biochemical testing, enabling timely intervention and improved patient outcomes
- For instance, next-generation sequencing (NGS) panels are increasingly being adopted in clinical laboratories to detect NEU1 mutations, facilitating precise diagnosis of both Sialidosis Type I and Type II
- Emerging diagnostic tools allow clinicians to identify sialidosis in asymptomatic or mildly affected patients, supporting early therapeutic planning and genetic counseling for affected families
- The integration of genetic screening with electronic health records (EHRs) and telemedicine platforms allows for centralized patient monitoring, longitudinal tracking, and better-informed clinical decision-making
- This trend towards early detection and integration of advanced diagnostics is reshaping patient management expectations, with companies such as BioMarin and Chiesi developing comprehensive genetic testing solutions and disease awareness programs
- Rising awareness of the benefits of early genetic screening among healthcare providers and families is driving adoption, ultimately supporting market growth in both developed and emerging regions
Sialidosis Market Dynamics
Driver
Rising Awareness and Support for Rare Disease Therapies
- The increasing awareness of rare lysosomal storage disorders and the expansion of supportive care programs are significant drivers for the heightened demand for sialidosis diagnostics and therapies
- For instance, in March 2025, Chiesi Group launched a patient support initiative in North America focusing on lysosomal storage disorders, enhancing access to genetic counseling and treatment monitoring services
- As healthcare providers and patient advocacy groups promote early detection and treatment options, demand for enzyme replacement therapies and supportive interventions is rising
- Furthermore, increased funding for rare disease research and government incentives for orphan drug development are encouraging innovation in sialidosis therapeutics
- The availability of patient assistance programs, combined with awareness campaigns and educational outreach, is improving treatment uptake and adherence among diagnosed patients
- Enhanced collaboration between pharmaceutical companies, clinics, and research institutions is further accelerating market growth in both diagnostics and treatment domains. Expansion of telehealth and remote monitoring services is improving disease management for patients in geographically underserved regions
- Growing partnerships between rare disease foundations and biotech firms are facilitating faster clinical trials and accelerated therapy development for sialidosis
Restraint/Challenge
High Treatment Costs and Limited Awareness in Emerging Regions
- The high costs associated with enzyme replacement therapy, genetic counseling, and supportive care pose a significant challenge to broader market adoption, particularly in price-sensitive regions
- For instance, the annual cost of specialized sialidosis therapies can exceed USD 200,000, making them inaccessible to many patients without insurance or government support
- Limited awareness of sialidosis among healthcare providers in emerging markets delays diagnosis and reduces treatment penetration, restricting overall market growth
- In addition, complex regulatory requirements for orphan drugs and genetic therapies can slow the approval and distribution process, increasing time-to-market for new treatments
- Overcoming these challenges requires enhanced patient education, subsidized therapy programs, and streamlined regulatory pathways to make sialidosis diagnostics and treatments more accessible
- Companies such as BioMarin and Chiesi are working on patient assistance and awareness initiatives, but high costs and limited regional awareness remain major adoption barriers. Challenges in long-term safety data and limited clinical trial populations for rare disease therapies may reduce physician confidence in prescribing new treatments
- Inadequate healthcare infrastructure and lack of reimbursement policies in certain emerging economies further limit patient access to sialidosis diagnostics and therapies
Sialidosis Market Scope
The market is segmented on the basis of type, treatment, diagnosis, symptoms, end-users, and distribution channel.
- By Type
On the basis of type, the sialidosis market is segmented into Sialidosis Type I and Sialidosis Type II. The Sialidosis Type I segment dominated the market with the largest revenue share of 52.8% in 2025, driven by its relatively milder progression, better diagnostic recognition, and increasing treatment availability. Patients with Type I typically have a later onset and slower disease progression, allowing for timely intervention and management through genetic counseling and supportive care. The segment benefits from growing awareness among clinicians and families, as well as improved early detection programs. Sialidosis Type I patients often respond well to symptomatic treatments such as anticonvulsants and vision therapy, which encourages higher adoption. The accessibility of diagnostic testing in developed countries has further contributed to this dominance. The increasing availability of patient support programs and educational initiatives has also boosted the segment’s market presence.
The Sialidosis Type II segment is expected to witness the fastest growth rate of 20.5% from 2026 to 2033, fueled by rising efforts in early detection and novel therapy development. Type II is more severe and has an earlier onset, historically limiting treatment options, but ongoing research in gene therapy and enzyme replacement is expanding intervention possibilities. Awareness campaigns and rare disease foundations are supporting families to recognize symptoms early. Improved access to healthcare infrastructure in emerging markets is helping more patients receive proper diagnosis. Hospitals and clinics are increasingly providing multidisciplinary care, which supports growth in Type II. Government incentives for orphan drug development further accelerate this segment’s expansion.
- By Treatment
On the basis of treatment, the sialidosis market is segmented into anticonvulsant, genetic counseling, and others. The Genetic Counseling segment dominated the market with the largest revenue share of 48.7% in 2025, as counseling is critical for early diagnosis, family planning, and guiding patients through disease management. Genetic counseling is increasingly integrated with diagnostic testing, allowing clinicians to recommend timely interventions. The segment benefits from rising awareness programs and rare disease support initiatives sponsored by pharmaceutical companies and nonprofit organizations. Comprehensive counseling helps patients understand treatment options, prognosis, and lifestyle adaptations. Access to counseling services is more established in developed countries, contributing to higher adoption. Ongoing training programs for clinicians also improve service availability and quality.
The Anticonvulsant segment is expected to witness the fastest growth rate of 22.1% from 2026 to 2033, driven by the high prevalence of seizures in sialidosis patients and the increasing availability of newer anticonvulsant therapies. Anticonvulsants help manage neurological symptoms, improving patient quality of life. Hospitals and clinics are adopting these therapies in outpatient and inpatient settings. Improved safety and efficacy profiles of new drugs encourage higher physician confidence. Expanding insurance coverage in certain regions is making anticonvulsants more accessible. Patient awareness of symptom management is also increasing adoption rates.
- By Diagnosis
On the basis of diagnosis, the sialidosis market is segmented into blood test, urine test, and skin biopsy. The Blood Test segment dominated the market with the largest revenue share of 50.2% in 2025, owing to its non-invasive nature and widespread use for early detection of lysosomal enzyme deficiencies. Blood-based diagnostics allow precise identification of NEU1 mutations and guide treatment planning. Blood tests are often the first line of investigation, followed by confirmatory tests, making them integral to the diagnostic workflow. Declining costs of advanced blood-based panels further drive adoption. Integration with electronic health records facilitates long-term patient monitoring. Healthcare providers increasingly recommend blood tests as part of newborn and pediatric screening programs.
The Urine Test segment is expected to witness the fastest CAGR of 21.8% from 2026 to 2033, fueled by its role in early disease detection and biochemical monitoring. Urine tests are non-invasive, making them ideal for pediatric patients. Ongoing research is improving the sensitivity and specificity of urine-based markers. The tests support disease monitoring and therapy evaluation over time. Telemedicine initiatives are enabling remote sample collection and reporting. Increasing awareness of non-invasive diagnostic methods in emerging markets supports rapid adoption.
- By Symptoms
On the basis of symptoms, the sialidosis market is segmented into gait disturbance, reduced visual acuity, myoclonus, ataxia, leg tremors, seizures, and others. The Reduced Visual Acuity segment dominated the market with a share of 46.5% in 2025, as ocular symptoms are often the first recognized manifestation of sialidosis. Patients require ophthalmologic interventions and supportive therapies to manage vision impairment. Early detection of visual problems allows timely genetic counseling and symptom management. Clinical programs emphasize monitoring for progressive vision loss. Supportive care, including corrective lenses and vision aids, increases treatment adoption. Awareness campaigns educate families on the importance of ophthalmologic evaluation.
The Seizures segment is expected to witness the fastest growth rate of 23.0% from 2026 to 2033, driven by increasing awareness of neurological complications and rising use of anticonvulsants. Effective seizure management significantly improves patient quality of life. Hospitals and clinics are increasingly adopting seizure monitoring and treatment programs. Novel anticonvulsants with better safety profiles are accelerating market adoption. Patient education on symptom management further drives growth. Telehealth and remote monitoring solutions also support the rising prevalence of treatment adoption.
- By End-Users
On the basis of end-users, the sialidosis market is segmented into clinic, hospital, and others. The Hospital segment dominated the market with the largest revenue share of 54.1% in 2025, as hospitals provide comprehensive diagnostic, therapeutic, and genetic counseling services. Hospitals offer multidisciplinary care essential for managing this complex rare disease. Advanced diagnostic tools and specialized treatments are primarily available in hospitals. Patient support programs and clinical trial access further increase adoption. Rising investment in rare disease centers strengthens market dominance. Hospitals remain the first point of contact for patients with severe symptoms.
The Clinic segment is expected to witness the fastest growth rate of 20.9% from 2026 to 2033, fueled by increasing outpatient management, early genetic counseling, and improved awareness among clinicians. Clinics provide accessible follow-up care and monitoring. Integration of telemedicine and genetic testing enhances patient reach. Clinics in urban and semi-urban regions are increasingly involved in rare disease care. Community health initiatives are expanding their adoption. Growing collaboration between clinics and hospitals is supporting comprehensive care delivery.
- By Distribution Channel
On the basis of distribution channel, the sialidosis market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The Hospital Pharmacy segment dominated the market with the largest revenue share of 49.8% in 2025, as hospital pharmacies ensure immediate access to specialized therapies alongside clinical care. They provide controlled dispensing of orphan drugs and integrate patient monitoring. Access to clinical trial medications further drives adoption. Hospitals often combine therapy with patient education and support programs. The hospital pharmacy network ensures adherence and follow-up. Increasing rare disease treatment centers in developed regions strengthen this segment.
The Online Pharmacy segment is expected to witness the fastest growth rate of 24.3% from 2026 to 2033, driven by rising e-commerce adoption and convenience for patients in remote regions. Online pharmacies enable home delivery of therapies and supportive medications. Telemedicine integration enhances prescription fulfillment. Patient awareness campaigns promote safe online access to therapies. Expanding internet access and digital literacy support growth in emerging markets. Online pharmacy solutions improve continuity of care for chronic symptom management.
Sialidosis Market Regional Analysis
- North America dominated the sialidosis market with the largest revenue share of 41.4% in 2025, driven by advanced healthcare infrastructure, high investment in rare disease research, and early adoption of genetic screening programs, with the U.S. leading in clinical trials and treatment access
- Patients and healthcare providers in the region highly value timely diagnosis, access to specialized therapies, and comprehensive genetic counseling services for effective disease management
- This widespread adoption is further supported by high healthcare expenditure, a well-established rare disease treatment ecosystem, and the presence of key market players actively investing in research and patient support programs, establishing North America as the leading region for sialidosis care
U.S. Sialidosis Market Insight
The U.S. sialidosis market captured the largest revenue share of 78% in 2025 within North America, fueled by advanced healthcare infrastructure and high awareness of rare genetic disorders. Patients increasingly prioritize early diagnosis through genetic screening and timely access to specialized therapies, including supportive care and counseling. The growing presence of rare disease centers and clinical trial programs further propels the market. Moreover, government incentives for orphan drug development and strong patient advocacy initiatives are significantly contributing to the market's expansion. The U.S. remains the primary hub for research and innovation in sialidosis therapies.
Europe Sialidosis Market Insight
The Europe sialidosis market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by increasing rare disease awareness and government support for orphan drug programs. The region’s robust healthcare infrastructure and emphasis on early genetic screening foster the adoption of sialidosis diagnostics and treatments. European patients also value comprehensive care services, including genetic counseling, anticonvulsant therapy, and supportive management. The market is witnessing growth across hospitals, clinics, and specialty centers, with increasing collaboration between healthcare providers and pharmaceutical companies.
U.K. Sialidosis Market Insight
The U.K. sialidosis market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by rising awareness of lysosomal storage disorders and demand for early diagnosis. Both healthcare providers and families increasingly prioritize genetic counseling and symptomatic management for patients. In addition, government initiatives supporting rare disease treatment, alongside strong healthcare delivery networks, are encouraging broader adoption. The U.K.’s integration of telemedicine and specialized clinics is further stimulating market growth. Research funding and patient advocacy programs also play a key role in expanding treatment access.
Germany Sialidosis Market Insight
The Germany sialidosis market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing awareness of rare genetic disorders and well-established healthcare systems. Germany’s focus on innovation, combined with advanced diagnostic facilities and hospital infrastructure, promotes adoption of early genetic testing and treatment options. Patients increasingly seek multidisciplinary care, including enzyme replacement, anticonvulsants, and supportive therapies. Collaboration between research institutions, hospitals, and pharmaceutical companies enhances clinical trial opportunities and access to novel therapies. The emphasis on patient-centered care aligns with local expectations and contributes to steady market growth.
Asia-Pacific Sialidosis Market Insight
The Asia-Pacific sialidosis market is poised to grow at the fastest CAGR of 25% during the forecast period of 2026 to 2033, driven by rising awareness of rare diseases, improving healthcare infrastructure, and expanding access to diagnostics and treatments. Countries such as China, Japan, and India are witnessing increased adoption of genetic screening and counseling services. Government initiatives supporting rare disease care and the expansion of specialty clinics are facilitating early diagnosis and treatment. Growing telemedicine penetration and the availability of affordable therapies are also accelerating market growth. The region’s large patient population and rising investment in rare disease research further support rapid expansion.
Japan Sialidosis Market Insight
The Japan sialidosis market is gaining momentum due to the country’s advanced healthcare system, high awareness of rare disorders, and strong focus on early genetic screening. Patients and healthcare providers emphasize timely diagnosis and access to enzyme replacement and supportive therapies. The integration of telemedicine and specialized rare disease centers is fueling growth. In addition, Japan’s aging population drives demand for easier-to-access genetic counseling and symptom management. Government support for orphan drug development further encourages adoption. Collaborative research programs between hospitals and pharmaceutical companies are also expanding therapeutic options.
India Sialidosis Market Insight
The India sialidosis market accounted for the largest market revenue share in Asia-Pacific in 2025, attributed to increasing rare disease awareness, expanding healthcare access, and improving diagnostic facilities. The growing number of specialty clinics and hospitals offering genetic screening and counseling supports early detection and treatment. India’s large population and rising middle-class healthcare spending are driving demand for affordable therapies. Government initiatives promoting rare disease care and telemedicine integration are facilitating access to treatment in both urban and semi-urban areas. Strong domestic pharmaceutical presence and patient support programs are key factors propelling market growth.
Sialidosis Market Share
The Sialidosis industry is primarily led by well-established companies, including:
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- Abbott (U.S.)
- GSK plc (U.K.)
- Takeda Pharmaceutical Company Limited (Japan)
- Sanofi (U.S.)
- BioMarin Pharmaceutical Inc. (U.S.)
- Amicus Therapeutics, Inc. (U.S.)
- Alexion Pharmaceuticals, Inc. (U.S.)
- Chiesi Farmaceutici SpA (Italy)
- Eli Lilly and Company (U.S.)
- Horizon Therapeutics Plc (Ireland)
- Actelion Pharmaceuticals Ltd (Switzerland)
- Leadient (U.S.)
- Orphazyme (Denmark)
- Recordati S.p.A (Italy)
- Sigilon Therapeutics, Inc. (U.S.)
- Viatris Inc. (U.S.)
What are the Recent Developments in Global Sialidosis Market?
- In June 2025, TFBS Bioscience in Taiwan partnered with Wuh‐Liang Hwu (MD, PhD) to launch a new gene‑therapy development project targeting Sialidosis using an AAV9 vector, with TFBS providing GMP‑grade manufacturing, quality and safety testing in support of a planned Phase I/II clinical trial. The partnership reflects growing global interest in translational gene therapies for Sialidosis and expands manufacturing capabilities in Asia
- In May 2024, a landmark pre clinical study titled “AAV mediated gene therapy for sialidosis” was published, demonstrating that co delivery of human NEU1 and its chaperone PPCA via scAAV2/8 in a Neu1⁻/⁻ mouse model restored NEU1 activity in brain, heart and visceral organs and reversed disease markers. The study marks a major step toward a first in human gene therapy for sialidosis
- In April 2024, Cummings School of Veterinary Medicine, Tufts University and UMass Chan Medical School announced a research collaboration (supported by a National Institutes of Health grant) to develop a sheep model of sialidosis, with the aim of advancing gene‑therapy treatments for human patients with the disease
- In May 2023, UMass Chan Medical School reported preliminary results of AAV‑based gene therapy vectors encoding NEU1 (sialidase) in preclinical models of sialidosis, indicating enzyme activity restoration and disease marker improvements in mice. The report marks a potential translational step toward human therapies for a disorder with no existing approved treatment
- In August 2023, Chiesi Global Rare Diseases entered into a co‑development agreement with Aliada Therapeutics to develop treatments for lysosomal storage disorders (LSDs) that can cross the blood‑brain barrier (BBB) a collaboration which is relevant to sialidosis given its CNS involvement
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.