Global Single Use Bioreactors For Vaccine Production Market
Market Size in USD Billion
CAGR :
% 
 USD
2.15 Billion
USD
5.98 Billion
2024
2032
USD
2.15 Billion
USD
5.98 Billion
2024
2032
| 2025 –2032 | |
| USD 2.15 Billion | |
| USD 5.98 Billion | |
|
|
|
|
Single-Use Bioreactors for Vaccine Production Market Size
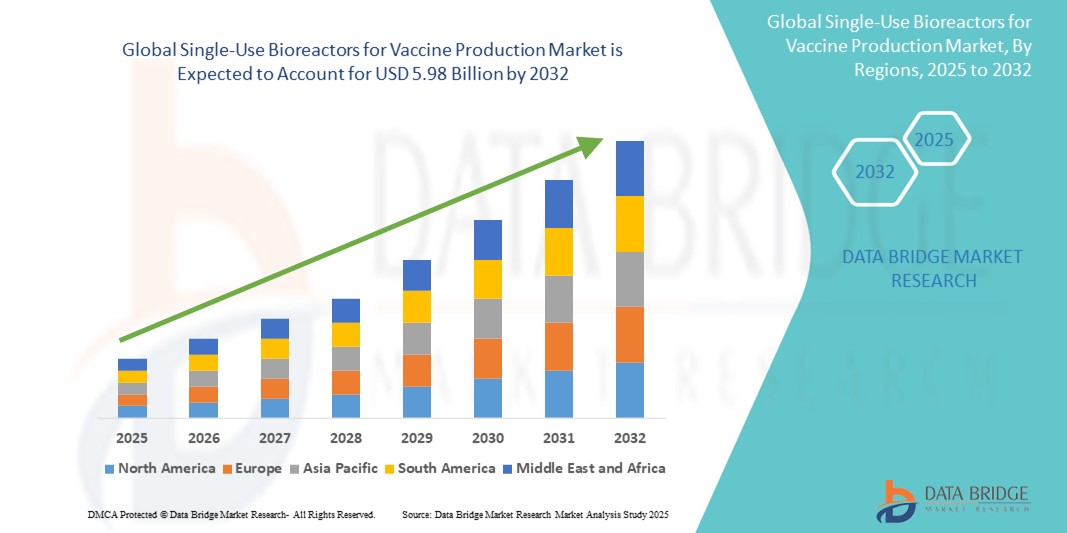
- The global single-use bioreactors for vaccine production market size was valued at USD 2.15 billion in 2024 and is expected to reach USD 5.98 billion by 2032, at a CAGR of 13.60% during the forecast period
- The market growth is largely fueled by the increasing demand for flexible, scalable, and contamination-free manufacturing technologies in vaccine production, particularly in response to global health emergencies and the expanding biologics pipeline
- Furthermore, the rise in contract manufacturing activities, coupled with rapid advancements in bioprocessing technologies and disposable systems, is positioning single-use bioreactors as a key solution in modern vaccine manufacturing. These driving factors are accelerating market adoption, thereby significantly boosting the industry's growth
Single-Use Bioreactors for Vaccine Production Market Analysis
- Single-use bioreactors, which enable disposable, sterile, and efficient cell culture environments, are becoming increasingly vital to vaccine manufacturing processes in both commercial and research settings due to their operational flexibility, reduced risk of cross-contamination, and compatibility with modular bioproduction systems
- The escalating demand for single-use bioreactors is primarily fueled by the rising global focus on rapid vaccine development, expanding biologics pipeline, and the need for cost-effective, scalable biomanufacturing platforms that support faster turnaround and regulatory compliance
- North America dominated the single-use bioreactors for vaccine production market with the largest revenue share of 41.8% in 2024, supported by its advanced biopharmaceutical infrastructure, strong government funding in public health initiatives, and the presence of leading industry players and CDMOs, particularly in the U.S., which has embraced single-use systems for both clinical and commercial vaccine production
- Asia-Pacific is expected to be the fastest growing region in the single-use bioreactors for vaccine production market during the forecast period due to expanding vaccine production capabilities, growing biotech investments, and supportive regulatory frameworks in countries such as China, India, and South Korea
- The single-use bioreactor systems segment dominated the single-use bioreactors for vaccine production market with a market share of 52.9% in 2024, owing to their central role in upstream processing, scalability, ease of integration into existing facilities, and widespread adoption across both R&D and commercial vaccine manufacturing workflows
Report Scope and Single-Use Bioreactors for Vaccine Production Market Segmentation
|
Attributes |
Single-Use Bioreactors for Vaccine Production Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Single-Use Bioreactors for Vaccine Production Market Trends
“Increased Adoption of Modular, Scalable Bioprocessing Systems”
- A significant and accelerating trend in the global single-use bioreactors for vaccine production market is the growing shift toward modular and scalable bioprocessing solutions, enabling faster setup, flexible production, and reduced contamination risks in both clinical and commercial vaccine manufacturing
- For instance, Sartorius’ BIOSTAT STR single-use bioreactor platform supports rapid deployment and scale-up from bench to production scale, allowing vaccine manufacturers to respond swiftly to outbreaks. Similarly, Thermo Fisher Scientific’s HyPerforma bioreactors are being adopted for their plug-and-play compatibility across upstream processes
- Modular systems integrated with single-use technologies allow manufacturers to tailor vaccine production volumes efficiently, minimize downtime between campaigns, and reduce the need for extensive cleaning and validation, accelerating time-to-market
- In addition, many platforms now support real-time data analytics, automation, and closed-system operations, which enhance process control and compliance with regulatory standards. Companies such as Cytiva and ABEC are investing in flexible manufacturing systems that accommodate a wide range of vaccine modalities including mRNA, viral vector, and protein subunit types
- This trend toward adaptable, ready-to-deploy manufacturing solutions is reshaping how both large biopharma firms and CDMOs approach vaccine production infrastructure. As global demand rises for rapid, scalable solutions, the adoption of advanced single-use bioreactor systems is becoming a cornerstone of modern vaccine manufacturing strategy
- The demand for flexible, high-efficiency bioreactor platforms is growing rapidly across both established and emerging markets, as governments and manufacturers prioritize fast, safe, and cost-effective vaccine production capabilities
Single-Use Bioreactors for Vaccine Production Market Dynamics
Driver
“Growing Vaccine Demand and Biopharma Investment in Scalable Manufacturing”
- The increasing global need for vaccines driven by emerging infectious diseases, population growth, and rising immunization efforts is a major driver for the adoption of single-use bioreactors, which offer fast, clean, and scalable manufacturing options
- For instance, in February 2024, Merck KGaA announced expansion of its Singapore bioprocessing facility with additional single-use systems to support vaccine production. Such initiatives from major players are expected to accelerate global market growth during the forecast period
- As both public and private sectors increase investments in vaccine development and biologics infrastructure, single-use bioreactors are becoming essential tools to meet high-throughput needs without the complexities of traditional stainless-steel systems
- These systems allow manufacturers to efficiently manage small batch sizes for personalized vaccines or scale up for large population needs, supporting both flexibility and speed in response to public health demands. The simplified validation, reduced downtime, and lower risk of contamination further contribute to the rising preference for single-use technologies in vaccine manufacturing
Restraint/Challenge
“High Operational Costs and Supply Chain Limitations”
- Despite their advantages, the relatively high cost of single-use bioreactor components and associated consumables remains a challenge, particularly for manufacturers in cost-sensitive or resource-limited regions
- In addition, the global supply chain for single-use components such as specialty media bags, connectors, and sterile filters can be vulnerable to disruption, as witnessed during the COVID-19 pandemic, where biopharma manufacturers experienced delays due to shortages
- For instance, several CDMOs in Europe and Asia reported postponed vaccine campaigns due to limited availability of single-use systems in early 2023, highlighting the need for robust supplier networks and inventory management
- Overcoming these challenges will require strategic supplier partnerships, localized manufacturing, and improved cost-efficiency through technology optimization
- Industry leaders are working on next-generation single-use systems that balance performance with affordability to enable broader adoption globally, especially among emerging-market producers and smaller biopharmaceutical firms
Single-Use Bioreactors for Vaccine Production Market Scope
The market is segmented on the basis of product type, reactor type, cell type, and end-user.
- By Product Type
On the basis of product type, the single-use bioreactors for vaccine production market is segmented into single-use bioreactor systems, media bags, filtration assemblies, and other accessories. The single-use bioreactor systems segment dominated the market with the largest market revenue share of 52.9% in 2024, driven by their core role in upstream vaccine production, scalability across different batch sizes, and ability to minimize contamination risk. These systems are highly favored in both clinical and commercial production due to their disposable nature, reduced cleaning requirements, and compatibility with automated platforms. Manufacturers often prioritize single-use bioreactor systems for their operational efficiency and faster turnaround times.
The media bags segment is anticipated to witness the fastest growth rate of 20.8% from 2025 to 2032, fueled by increasing demand for pre-sterilized consumables and the shift toward closed, aseptic processing environments. Media bags support the safe storage and handling of culture media and process fluids, contributing to the broader adoption of disposable technologies in vaccine manufacturing setups.
- By Reactor Type
On the basis of reactor type, the single-use bioreactors for vaccine production market is segmented into stirred-tank bioreactors, wave-induced bioreactors, bubble-column bioreactors, and other types. The stirred-tank bioreactors segment dominated the market with the largest market revenue share in 2024, driven by their established use in high-density cell cultures and large-scale vaccine production. Stirred-tank designs offer superior mixing, gas transfer efficiency, and scalability, making them ideal for producing a wide range of vaccines using mammalian, bacterial, or yeast cells. The flexibility to integrate process control technologies further enhances their performance in GMP environments.
The wave-induced bioreactors segment is anticipated to witness the fastest growth rate of 21.5% from 2025 to 2032, fueled by growing adoption in small-batch and early-phase vaccine development. These bioreactors provide gentle agitation suitable for shear-sensitive cells and are increasingly used in modular and mobile vaccine manufacturing facilities due to their compact footprint and rapid setup.
- By Cell Type
On the basis of cell type, the single-use bioreactors for vaccine production market is segmented into mammalian cells, bacterial cells, yeast cells, and other cells. The mammalian cells segment dominated the market with the largest market revenue share of 58.3% in 2024, driven by their essential role in producing viral vector and recombinant protein-based vaccines. Commonly used cell lines such as CHO, Vero, and HEK293 offer high productivity and safety profiles required for regulated vaccine production. The segment continues to benefit from innovation in cell line engineering and bioprocess optimization.
The bacterial cells segment is anticipated to witness the fastest growth rate of 19.6% from 2025 to 2032, fueled by rising demand for recombinant protein and subunit vaccines. Bacterial platforms such as E. coli offer high yield, cost-effectiveness, and faster expression cycles, making them attractive for both established and emerging vaccine developers. The simplicity of bacterial systems supports broader global access and scalable production.
- By End-User
On the basis of end-user, the single-use bioreactors for vaccine production market is segmented into pharmaceutical & biopharmaceutical companies, contract manufacturing organizations, academic & research institutes, and others. The pharmaceutical & biopharmaceutical companies segment dominated the market with the largest market revenue share of 49.6% in 2024, driven by in-house vaccine R&D, growing biologics pipelines, and the push for flexible GMP-compliant manufacturing solutions. These companies are increasingly adopting single-use technologies to reduce downtime, enhance process control, and accelerate time-to-market for vaccine candidates.
The contract manufacturing organizations segment is anticipated to witness the fastest growth rate of 22.3% from 2025 to 2032, fueled by increased outsourcing of vaccine production by small and mid-sized biotech firms. CMOs benefit from the modularity, cost-efficiency, and speed of deployment offered by single-use bioreactors, making them ideal partners for scalable and compliant vaccine manufacturing across various modalities.
Single-Use Bioreactors for Vaccine Production Market Regional Analysis
- North America dominated the single-use bioreactors for vaccine production market with the largest revenue share of 41.8% in 2024, supported by its advanced biopharmaceutical infrastructure, strong government funding in public health initiatives, and the presence of leading industry players and CDMOs
- The region’s leadership is supported by the widespread adoption of innovative bioprocessing technologies and the presence of major pharmaceutical companies, contract manufacturing organizations, and leading equipment providers
- High regulatory standards, coupled with robust government and private funding for vaccine development, further contribute to the region’s dominance, positioning single-use bioreactors as a preferred solution for both clinical and commercial-scale vaccine manufacturing
U.S. Single-Use Bioreactors for Vaccine Production Market Insight
The U.S. single-use bioreactors for vaccine production market captured the largest revenue share of 79.2% in 2024 within North America, fueled by strong government and private investments in vaccine R&D and the rapid advancement of biomanufacturing infrastructure. The country is a global hub for vaccine development, with key pharmaceutical companies and CDMOs actively adopting single-use technologies for both clinical trials and commercial-scale production. The growing focus on personalized vaccines, automation, and speed-to-market continues to drive demand for scalable, flexible bioprocessing solutions such as single-use bioreactors.
Europe Single-Use Bioreactors for Vaccine Production Market Insight
The Europe single-use bioreactors for vaccine production market is projected to grow at a substantial CAGR throughout the forecast period, primarily driven by strong pharmaceutical R&D capabilities and increasing adoption of biopharmaceutical production technologies. Regulatory support for biologics, coupled with rising demand for efficient and clean vaccine manufacturing systems, is propelling the uptake of single-use bioreactors. The market is expanding across key applications in national immunization programs, commercial-scale facilities, and academic collaborations, particularly in Germany, France, and Switzerland.
U.K. Single-Use Bioreactors for Vaccine Production Market Insight
The U.K. single-use bioreactors for vaccine production market is anticipated to grow at a noteworthy CAGR during the forecast period, supported by robust government funding in life sciences and innovation in vaccine manufacturing. The country’s biotech ecosystem is increasingly embracing modular, single-use technologies to support rapid and localized vaccine production. Initiatives such as the UK Life Sciences Vision and support for pandemic preparedness continue to strengthen the market outlook for disposable bioreactor systems.
Germany Single-Use Bioreactors for Vaccine Production Market Insight
The Germany single-use bioreactors for vaccine production market is expected to expand at a considerable CAGR during the forecast period, driven by its advanced pharmaceutical manufacturing landscape and strong emphasis on bioprocess innovation. German companies are actively investing in GMP-compliant facilities and upgrading production lines with single-use systems to enhance flexibility and reduce contamination risk. The focus on sustainability and precision in vaccine production further aligns with the advantages offered by single-use bioreactors.
Asia-Pacific Single-Use Bioreactors for Vaccine Production Market Insight
The Asia-Pacific single-use bioreactors for vaccine production market is poised to grow at the fastest CAGR of 24.7% from 2025 to 2032, driven by rapid urbanization, expanding biotech hubs, and strong government initiatives to promote vaccine self-sufficiency in countries such as China, India, South Korea, and Japan. The increasing need for cost-effective, flexible manufacturing platforms is boosting the appeal of single-use technologies across public health and commercial sectors. Local manufacturing of bioreactor components and rising investments in healthcare infrastructure are further accelerating market expansion.
Japan Single-Use Bioreactors for Vaccine Production Market Insight
The Japan single-use bioreactors for vaccine production market is gaining traction due to its focus on innovation, digital biomanufacturing, and rapid vaccine delivery capabilities. Japan’s strong biopharma sector is increasingly adopting single-use technologies to support flexible, high-quality production in response to pandemic readiness and aging population needs. Integration of advanced automation and analytics within single-use systems is contributing to process efficiency and product consistency in the country’s vaccine supply chain.
India Single-Use Bioreactors for Vaccine Production Market Insight
The India single-use bioreactors for vaccine production market accounted for the largest revenue share within Asia-Pacific in 2024, attributed to expanding domestic vaccine production, growing biotech startups, and strong government support through initiatives such as “Make in India” and “Ayushman Bharat.” India’s rising demand for low-cost, high-efficiency bioproduction platforms is driving the uptake of single-use bioreactors across government programs and private manufacturers. The country's position as a global vaccine supplier continues to spur demand for rapid, scalable manufacturing solutions.
Single-Use Bioreactors for Vaccine Production Market Share
The single-use bioreactors for vaccine production industry is primarily led by well-established companies, including:
- Thermo Fisher Scientific Inc. (U.S.)
- Danaher Corporation (U.S.)
- Merck KGaA (Germany)
- Sartorius AG (Germany)
- Eppendorf SE (Germany)
- Avantor, Inc. (U.S.)
- Corning Incorporated (U.S.)
- PBS Biotech, Inc. (U.S.)
- ABEC, Inc. (U.S.)
- Meissner Filtration Products, Inc. (U.S.)
- Applikon Biotechnology B.V. (Netherlands)
- Distek, Inc. (U.S.)
- Cellexus Ltd. (U.K.)
- Getinge AB (Sweden)
- Pierre Guérin Technologies (France)
- ZETA Holding GmbH (Austria)
- Stobbe Pharma GmbH (Germany)
- Solaris Biotechnology Srl (Italy)
- Entegris, Inc. (U.S.)
What are the Recent Developments in Global Single-Use Bioreactors for Vaccine Production Market?
- In April 2024, Thermo Fisher Scientific announced the expansion of its St. Louis biologics facility with new single-use bioreactor lines specifically designed for vaccine and viral vector production. This strategic investment enhances the company’s global capacity to support both clinical and commercial vaccine manufacturing, underscoring its commitment to scalable, flexible solutions that accelerate time-to-market and reduce contamination risks in biologics production
- In March 2024, Cytiva launched the Xcellerex XDR-50 MO, a next-generation modular single-use bioreactor designed to streamline upstream processing for vaccine production. With improved process control and automation features, this system is aimed at supporting both research-scale and GMP-compliant vaccine manufacturing. The innovation reinforces Cytiva’s position as a key enabler of rapid, reliable bioproduction technologies
- In February 2024, Merck KGaA, Darmstadt, Germany (operating as MilliporeSigma in the U.S. and Canada), opened a new single-use manufacturing center in Molsheim, France. This facility focuses on producing single-use assemblies and bioreactors used in vaccine development and biopharmaceutical applications. The move addresses the increasing global demand for disposable bioprocessing tools, especially in pandemic preparedness and routine immunization programs
- In January 2024, Sartorius expanded its product portfolio with the introduction of the BIOSTAT STR® Generation 4 single-use bioreactor system, featuring enhanced digital integration, AI-assisted analytics, and advanced automation. Tailored for vaccine developers, this system is designed to optimize cell culture performance and reduce process variability, aligning with industry needs for precision and speed in biologics manufacturing
- In December 2023, ABEC, a global supplier of integrated solutions for biopharmaceutical manufacturing, completed the installation of custom single-use bioreactor systems for a leading vaccine developer in Southeast Asia. This project supports localized vaccine production and demonstrates ABEC’s flexibility in delivering scalable single-use solutions for emerging markets, reinforcing its global reach and adaptability to client-specific manufacturing goals
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.