Global Sirolimus Market
Market Size in USD Million
CAGR :
% 
 USD
231.61 Million
USD
331.26 Million
2024
2032
USD
231.61 Million
USD
331.26 Million
2024
2032
| 2025 –2032 | |
| USD 231.61 Million | |
| USD 331.26 Million | |
|
|
|
|
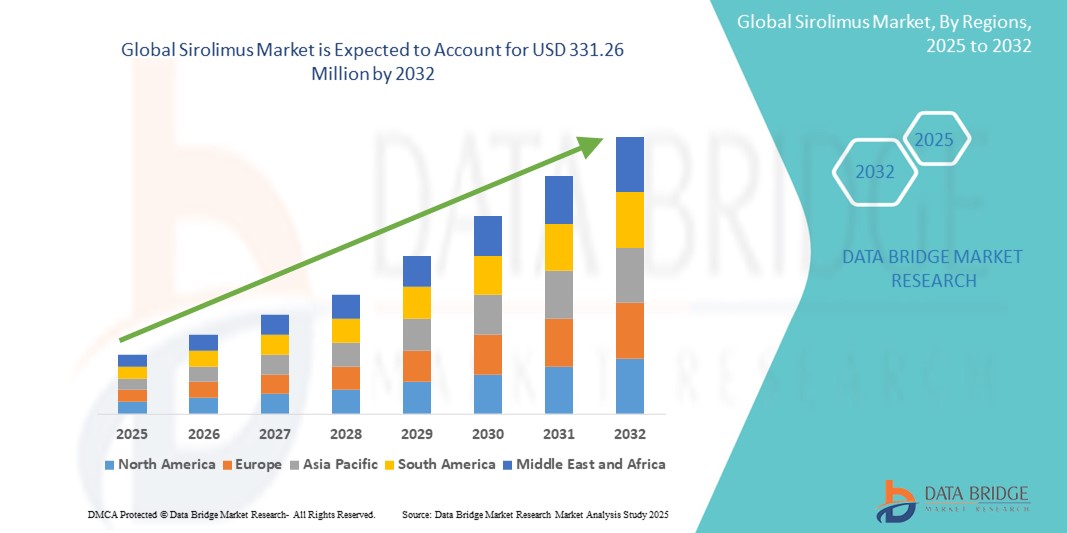
Sirolimus Market Size
- The global sirolimus market size was valued at USD 231.16 Million in 2024 and is expected to reach USD 331.26 Million by 2032, at a CAGR of 4.6% during the forecast period
- The market growth is largely fuelled by the increasing prevalence of chronic conditions such as organ transplant rejection, lymphangioleiomyomatosis (LAM), and certain cancers, which has led to a surge in demand for effective immunosuppressive therapies such as sirolimus. The drug’s proven efficacy in preventing organ rejection and treating rare diseases is reinforcing its critical role in modern therapeutic regimens
- Furthermore, ongoing advancements in drug formulation and targeted drug delivery technologies, along with increasing R&D investments by pharmaceutical companies, are expanding the clinical applications of sirolimus beyond traditional organ transplantation. These converging factors are accelerating the adoption of sirolimus-based therapies, thereby significantly boosting the growth of the global sirolimus market
Sirolimus Market Analysis
- Sirolimus, a potent immunosuppressant and mTOR inhibitor, is increasingly vital in modern medicine, primarily for preventing organ transplant rejection and treating specific rare diseases such as Lymphangioleiomyomatosis (LAM). Its anti-proliferative properties also make it significant in cancer research and in drug-eluting devices
- The escalating demand for sirolimus is primarily fuelled by the rising global number of organ transplantation procedures, the expanding applications in oncology and rare diseases, and continuous advancements in drug delivery systems
- North America dominated the sirolimus market with the largest revenue share, accounting for 39% in 2024. This dominance is characterized by advanced healthcare infrastructure, a high volume of organ transplant procedures, favorable reimbursement policies, and a strong presence of key pharmaceutical companies engaged in Sirolimus research and development
- Asia-Pacific is expected to be the fastest-growing region in the sirolimus market during the forecast period, with a projected CAGR of 6.84%. This rapid growth is driven by increasing healthcare expenditure, improving healthcare infrastructure, a rising number of organ transplant procedures, and a growing patient pool for associated conditions in countries such as China and India
- The organ transplant rejection segment dominated the sirolimus market, holding a market share of 52.7% in 2024. This is driven by its critical role as a key immunosuppressant to ensure successful patient outcomes post-transplantation, particularly in kidney and liver transplants. The drug’s well-established efficacy with minimal nephrotoxicity makes it a preferred choice among healthcare providers
Report Scope and Sirolimus Market Segmentation
|
Attributes |
Sirolimus Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Sirolimus Market Trends
“Enhanced Efficacy and Patient Management Through AI and Data Analytics”
- A significant and accelerating trend impacting the global sirolimus market is the deepening integration of artificial intelligence (AI) and advanced data analytics. This fusion of technologies is significantly enhancing drug discovery, personalized dosing, and patient management, ultimately improving therapeutic outcomes for patients on Sirolimus
- For instance, in 2024-2025, academic and pharmaceutical research is leveraging AI-powered models to personalize immunosuppressant drug dosing, including Sirolimus. Projects, such as those at Carleton University, are developing AI-based models to predict individual patient responses to drugs such as Sirolimus and tacrolimus, optimizing dosage and reducing risks of rejection or toxicity. This aims to move beyond trial-and-error, improving patient safety and treatment effectiveness
- AI integration in the Sirolimus market enables features such as predicting patient response to therapy, identifying optimal dosing regimens, and analyzing vast genomic and clinical datasets to uncover new indications or biomarkers. For instance, AI algorithms can analyze complex biological data to predict which patients with certain cancers or rare diseases are most likely to respond to Sirolimus. Furthermore, AI can aid in monitoring potential drug interactions and side effects by analyzing real-world patient data, enhancing safety
- The seamless integration of AI and data analytics in the drug development pipeline facilitates more efficient and targeted research. Through sophisticated algorithms, researchers can analyze patient data and preclinical models to streamline drug discovery processes, potentially reducing development costs and timelines for new Sirolimus formulations or applications by an estimated 25-50%
- This trend towards more intelligent, data-driven, and personalized therapeutic approaches is fundamentally reshaping expectations for how drugs such as Sirolimus are discovered, prescribed, and managed. Consequently, pharmaceutical companies and research institutions are investing heavily in AI platforms to accelerate clinical trials and refine treatment protocols
- The demand for AI-driven solutions that offer enhanced therapeutic efficacy and patient safety for drugs such as Sirolimus is growing rapidly across both clinical and research sectors, as healthcare providers increasingly prioritize personalized medicine and optimized patient outcomes
Sirolimus Market Dynamics
Driver
“Growing Incidence of Chronic Diseases, Organ Transplants, and Expanding Therapeutic Applications”
- The increasing global incidence of chronic diseases requiring immunosuppression, coupled with the accelerating number of organ transplantation procedures and the expansion of Sirolimus's therapeutic applications into new areas such as oncology and rare diseases, is a significant driver for the heightened demand in the Sirolimus market
- For instance, the rising burden of end-stage organ diseases, such as kidney, liver, and heart failure, directly translates into a greater need for immunosuppressants such as Sirolimus post-transplant. Furthermore, the growing recognition of Sirolimus's efficacy in treating rare conditions such as Lymphangioleiomyomatosis (LAM) and its exploration in various cancer therapies are opening new avenues for market growth. Such advancements and expanding clinical utility are expected to drive the Sirolimus industry growth in the forecast period
- As healthcare providers and patients become more aware of Sirolimus's multifaceted benefits – from effective immunosuppression with a favorable side effect profile in some cases, to its anti-proliferative effects – it offers a compelling therapeutic option over traditional mechanical approaches or less targeted drugs
- Furthermore, the continuous research and development into novel formulations and drug delivery systems for Sirolimus are making it an integral component of modern medical interventions, offering seamless integration with other treatment protocols and devices
- The convenience of a proven therapeutic agent for long-term management of chronic conditions, its potential in preventing graft-versus-host disease, and the ability to manage complex disease states through targeted mTOR inhibition are key factors propelling the adoption of Sirolimus in both hospital and outpatient settings. The trend towards personalized medicine and the increasing availability of generic Sirolimus options further contribute to market growth
Restraint/Challenge
“Concerns Regarding Side Effects, Drug Interactions, and High Treatment Costs”
- Concerns surrounding the significant side effects associated with Sirolimus, its complex drug interaction profile, and the relatively high initial and long-term treatment costs pose a significant challenge to broader market penetration and patient adherence. As Sirolimus relies on careful patient monitoring and management to balance efficacy with safety, its adverse event profile raises anxieties among potential prescribers and patients
- For instance, high-profile reports of potential side effects such as hyperlipidemia, proteinuria, delayed wound healing, and lung toxicity can make some physicians hesitant to prescribe Sirolimus as a first-line agent, particularly in certain patient populations. In addition, its narrow therapeutic window necessitates rigorous therapeutic drug monitoring, adding to healthcare costs and patient burden
- Addressing these safety and management concerns through robust patient education, pharmacogenomic testing to personalize dosing, and the development of formulations with improved side effect profiles is crucial for building prescriber and patient trust. Companies are investing in research to mitigate these issues and improve patient outcomes.In addition, the relatively high initial cost of Sirolimus (especially branded versions) and the ongoing costs of monitoring can be a barrier to adoption for price-sensitive healthcare systems and patients, particularly in developing regions or for those with limited insurance coverage. While generic versions of Sirolimus have become more affordable, the overall cost of complex treatment regimens and associated monitoring can still be substantial.
- While research is gradually leading to better management strategies for side effects and a better understanding of drug interactions, the perceived complexity of management can still hinder widespread adoption, especially for those healthcare providers who do not have immediate access to specialized monitoring facilities or expertise
Sirolimus Market Scope
The market is segmented on the basis of strength, application, drug class, dosage, route of administration and end user.
• By Strength
On the basis of strength, the sirolimus market is segmented into 0.5 mg, 1 mg, 2 mg, and 1 mg/mL. The 1 mg segment dominated the market with the largest revenue share of 48.5% in 2024, attributed to its standard dosing in maintenance immunosuppressive therapy following organ transplantation. Its widespread availability in tablet form and established efficacy contribute to its dominance.
The 1 mg/mL formulation is expected to witness the fastest CAGR of 20.4% from 2025 to 2032, driven by growing demand for intravenous delivery in cases requiring precise titration and pediatric use, as well as increasing adoption in hospital and critical care settings.
• By Application
On the basis of application, the sirolimus market is segmented into organ transplant rejection, sirolimus catheter device, sirolimus coated balloons, and others. The organ transplant rejection segment held the largest market revenue share of 52.7% in 2024, owing to the drug’s well-established role in preventing rejection in kidney and liver transplants. It is favored for its immunosuppressive efficacy with minimal nephrotoxicity.
The sirolimus coated balloons segment is expected to register the fastest CAGR of 18.9% from 2025 to 2032, driven by increasing clinical research and commercialization efforts in cardiovascular interventions for peripheral artery disease and in-stent restenosis.
• By Drug Class
On the basis of drug class, the sirolimus market is segmented into mTOR Inhibitors and selective immunosuppressants. The mTOR inhibitors segment accounted for the largest revenue share of 69.1% in 2024, as Sirolimus is primarily classified under this class, recognized for inhibiting T-cell proliferation and aiding long-term graft survival.
The selective immunosuppressants segment is anticipated to witness the fastest CAGR of 15.6% from 2025 to 2032, due to increasing interest in targeted immunomodulation with fewer systemic side effects, supporting broader applications in autoimmune and inflammatory conditions.
• By Dosage
On the basis of dosage, the sirolimus market is segmented into injection, tablet, and others. The tablet segment dominated the market with a share of 63.4% in 2024, driven by patient convenience, ease of dosing, and long-term maintenance therapy for transplant patients.
The injection segment is projected to grow at the fastest CAGR of 17.3% from 2025 to 2032, due to its rising use in acute care settings and precision dosing in specific clinical scenarios.
• By Route of Administration
On the basis of route of administration, the sirolimus market is segmented into oral, intravenous, and others. The oral route held the highest share of 67.8% in 2024, supported by the dominance of oral tablet formulations used in outpatient transplant care and long-term therapy.
The intravenous segment is expected to register the fastest CAGR of 16.4% from 2025 to 2032, reflecting increased hospital use, faster onset of action in emergencies, and new drug delivery advancements.
• By End Users
On the basis of end users, the sirolimus market is segmented into hospitals, specialty clinics, homecare, and others. The hospitals segment accounted for the largest market share of 55.6% in 2024, owing to the high volume of transplant surgeries, immediate postoperative care, and availability of infusion therapy.
The homecare segment is expected to grow at the fastest CAGR of 19.1% from 2025 to 2032, driven by the increasing use of oral therapies, telemedicine support, and preference for at-home monitoring in chronic transplant maintenance therapy.
• By Distribution Channel
On the basis of distribution channel, the sirolimus market is segmented into hospital pharmacy, retail pharmacy, online pharmacy, and others. The hospital pharmacy segment held the largest market revenue share of 46.9% in 2024, as it remains the primary distribution point for transplant-related medications during inpatient stays.
The online pharmacy segment is projected to experience the fastest CAGR of 21.5% from 2025 to 2032, driven by the rise in e-prescriptions, convenience in medication delivery, and the expanding digital health ecosystem supporting remote patient care.
Sirolimus Market Regional Analysis
- North America dominated the sirolimus market with the largest revenue share of 39% in 2024, driven by a high prevalence of organ transplant surgeries, strong healthcare infrastructure, and early adoption of advanced immunosuppressive therapies
- The region benefits from well-established pharmaceutical distribution networks and ongoing clinical trials focusing on mTOR inhibitors for various applications, including cardiovascular and oncology
- High healthcare spending, favorable reimbursement policies, and FDA approvals for Sirolimus-based drug-eluting devices contribute to continued market leadership
U.S. Sirolimus Market Insight
The U.S. sirolimus market captured 81.02% of the North America sirolimus market in 2024, owing to the high rate of organ transplants, advanced hospital facilities, and increasing off-label use of Sirolimus in oncology and dermatology. Strong R&D presence, particularly among major pharmaceutical companies and academic institutions, is propelling innovation in Sirolimus formulations. In addition, rising demand for personalized medicine, an aging population, and an increasing focus on biologics and targeted therapies fuel continued market expansion in the U.S.
Europe Sirolimus Market Insight
The Europe sirolimus market is projected to grow at a robust CAGR from 2025 to 2032, driven by the growing number of renal transplants, high awareness of immunosuppressive therapy, and support from centralized healthcare systems. The implementation of EU regulations encouraging drug safety, along with investments in drug-coated devices, enhances the growth outlook. European countries are also embracing Sirolimus in cardiovascular therapies, especially in stent coatings and drug-eluting balloons for peripheral arterial disease.
U.K. Sirolimus Market Insight
The U.K. sirolimus market is expected to grow at a noteworthy CAGR during the forecast period, supported by the NHS’s increasing procurement of immunosuppressants and ongoing initiatives to improve transplant outcomes. Rising concern for graft survival and cost-effectiveness has made Sirolimus an appealing alternative to calcineurin inhibitors. Furthermore, the expansion of clinical trials and fast-track approvals for orphan indications are accelerating the market trajectory.
Germany Sirolimus Market Insight
The Germany sirolimus market is projected to expand at a considerable CAGR from 2025 to 2032, due to increasing prevalence of chronic kidney and heart conditions, and a steady rise in transplant procedures. Germany’s strong pharmaceutical manufacturing base and emphasis on biosimilar development are facilitating access to Sirolimus-based therapies. Patient preference for oral formulations and rising demand for outpatient immunosuppressive therapy further fuel market demand.
Asia-Pacific Sirolimus Market Insight
The Asia-Pacific sirolimus market is poised to grow at the fastest CAGR of 6.84% during the forecast period, fueled by rising healthcare expenditure, expanding transplant programs, and increasing awareness of immunosuppressive drugs. Countries such as China and India are witnessing growing demand due to large patient pools, improving access to healthcare, and an expanding pharmaceutical manufacturing sector. In addition, government support for generic production and clinical research programs is boosting the availability of cost-effective Sirolimus formulations.
Japan Sirolimus Market Insight
The Japan sirolimus market is gaining significant momentum from 2025 to 2032, owing to the country’s advanced healthcare infrastructure and high incidence of chronic diseases requiring immunosuppression. Japan has also seen a rise in the use of Sirolimus in drug-eluting devices and ophthalmic applications. The government's push toward aging population care, personalized therapies, and targeted drug delivery is further stimulating market growth.
China Sirolimus Market Insight
The China sirolimus market held the largest revenue share in the Asia-Pacific sirolimus market in 2024, accounting for 38.5% of the regional market, driven by an expanding middle class, large transplant patient base, and government support for generic production. Rapid urbanization, high disease burden, and increasing penetration of Sirolimus in cardiovascular and oncology applications are key contributors. The rise of domestic pharmaceutical companies and export-oriented manufacturing enhances China’s position in both local and global Sirolimus supply chains.
Sirolimus Market Share
The sirolimus industry is primarily led by well-established companies, including:
- Accord Healthcare (U.S.)
- Apotex Inc. (Canada)
- Amneal Pharmaceuticals LLC. (U.S.)
- Pfizer Inc. (U.S.)
- Zydus Cadila (India)
- Dr. Reddy's Laboratories Ltd. (India)
- Torrent Pharmaceuticals Ltd. (India)
- Biocon (India)
- Concept Medical (U.S.)
- Intas Pharmaceuticals Ltd (India)
- Concord Biotech (India)
- Livzon (China)
- Actiza Pharmaceutical Private Limited (India)
- Tiefenbacher API + Ingredients GmbH & Co. KG (Germany)
- Delphis Pharmaceutical (India)
Latest Developments in Global Sirolimus Market
- In February 2023, Zydus Group obtained final approval from the U.S. Food and Drug Administration (FDA) for Sirolimus Tablets, 1 mg, and 2 mg (generic for Rapamune Tablets). This approval reinforced Zydus's product portfolio and market position, enabling the company to provide a vital medication for preventing organ rejection in kidney transplant recipients, enhancing affordability and access
- In January 2024 (published February 2024), a prospective clinical trial demonstrated the efficacy of Sirolimus in treating intractable lymphatic anomalies (LAs). This open-label, single-arm, multicenter study conducted in Japan showed that Sirolimus can reduce lymphatic tissue volume in LAs and may lead to improvements in clinical symptoms and quality of life. This highlights the ongoing expansion of Sirolimus's applications in rare and complex diseases beyond its traditional uses
- In April 2022 (with ongoing impact into 2023-2024), the FDA approved topical Sirolimus (HYFTOR) for facial angiofibroma associated with tuberous sclerosis complex. This marked the first topical treatment approved in the US for this specific manifestation. While the approval was earlier, its market adoption and the ongoing clinical experience continue to shape the Sirolimus landscape, highlighting the potential for localized formulations.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL SIROLIMUS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE SIROLIMUS MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL TRANSDERMAL PATCHE MARKET : RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES MODEL
5 INDUSTRY INSIGHTS
5.1 PATENT ANALYSIS
5.1.1 PATENT STRENGTH AND QUALITY
5.1.2 PATENT LITIGATION AND LICENSING
5.2 DRUG TREATMENT RATE BY MATURED MARKETS
5.3 DEMOGRAPHIC TRENDS
5.4 KEY PRICING STRATEGIES
5.5 KEY PATIENT ENROLLMENT STRATEGIES
5.6 INTERVIEWS WITH SPECIALIST
5.7 OTHER KOL SNAPSHOTS
6 EPIDEMIOLOGY
7 MERGERS AND ACQUISITION
7.1 LICENSING
7.2 COMMERCIALIZATION AGREEMENTS
8 REGULATORY FRAMEWORK
8.1 REGULATORY APPROVAL PROCESS
8.2 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
8.3 REGULATORY APPROVAL PATHWAYS
8.4 LICENSING AND REGISTRATION
8.5 POST-MARKETING SURVEILLANCE
8.6 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
9 PIPELINE ANALYSIS
9.1 CLINICAL TRIALS AND PHASE ANALYSIS
9.2 DRUG THERAPY PIPELINE
9.3 PHASE III CANDIDATES
9.4 PHASE II CANDIDATES
9.5 PHASE I CANDIDATES
9.6 OTHERS (PRE-CLINICAL AND RESEARCH)
10 MARKETED DRUG ANALYSIS
10.1 DRUG
10.1.1 BRAND NAME
10.1.2 GENERICS NAME
10.2 PHARACOLOGICAL CLASS OF THE DRUG
10.3 DRUG PRIMARY INDICATION
10.4 MARKET STATUS
10.5 MEDICATION TYPE
10.6 DRUG DOSAGES FORM
10.7 DOSAGES AVAILABILITY
10.8 PACKAGING TYPE
10.9 DRUG ROUTE OF ADMINISTRATION
10.1 DOSING FREQUENCY
10.11 DRUG INSIGHT
10.12 AN OVERVIEW OF THE DRUG DEVELOPMENT ACTIVITIES SUCH AS REGULATORY MILSTONE, SAFETY DATA AND EFFICACY DATA, MARKET EXCLUSIVITY DATA.
10.12.1 FORECAST MARKET OUTLOOK
10.12.2 CROSS COMPETITION
10.12.3 THERAPEUTIC PORTFOLIO
10.12.4 CURRENT DEVELOPMENT SCENARIO
11 MARKET ACCESS
11.1 10-YEAR MARKET FORECAST
11.2 CLINICAL TRIAL RECENT UPDATES
11.3 ANNUAL NEW FDA APPROVED DRUGS
11.4 DRUGS MANUFACTURER AND DEALS
11.5 MAJOR DRUG UPTAKE
11.6 CURRENT TREATMENT PRACTICES
11.7 IMPACT OF UPCOMING THERAPY
12 R & D ANALYSIS
12.1 COMPARATIVE ANALYSIS
12.2 DRUG DEVELOPMENTAL LANDSCAPE
12.3 IN-DEPTH INSIGHTS ON REGULATORY MILESTONES
12.4 THERAPEUTIC ASSESSMENT
12.5 ASSET-BASED COLLABORATIONS AND PARTNERSHIPS
13 MARKET OVERVIEW
13.1 DRIVERS
13.2 RESTRAINTS
13.3 OPPORTUNITIES
13.4 CHALLENGES
14 GLOBAL SIROLIMUS MARKET , SWOT AND DBMR ANALYSIS
15 GLOBAL SIROLIMUS MARKET, BY STRENGTH
15.1 OVERVIRE
15.2 0.5 MG
15.3 1MG
15.4 2MG
15.5 5 MG
15.6 OTHERS
16 GLOBAL SIROLIMUS MARKET, BY DRUG TYPE
16.1 OVERVIEW
16.2 BRANDED
16.2.1 RAPAMUNE
16.2.2 FYARRO
16.2.3 HYFTOR
16.2.4 OTHERS
16.3 GENERIC
17 GLOBAL SIROLIMUS MARKET, BY DOSAGE FORM
17.1 OVERVIEW
17.2 TABLETS
17.3 INJECTIONS
17.4 GEL
17.5 POWDER
17.6 OTHERS
18 GLOBAL SIROLIMUS MARKET, BY METHOD OF ADMINISTRATION
18.1 OVERVIEW
18.2 INTRAVENOUS
18.3 ORAL
18.4 TOPICAL
18.5 OTHERS
19 GLOBAL SIROLIMUS MARKET, BY APPLICATION
19.1 OVERVIEW
19.2 ORGAN TRANSPLANT REJECTIONS
19.2.1 LOW- TO MODERATE-IMMUNOLOGIC RISK
19.2.2 HIGH-IMMUNOLOGIC RISK
19.3 TUMOR/CANCER
19.3.1 UNRESECTABLE OR METASTATIC MALIGNANT PERIVASCULAR EPITHELIOID CELL TUMOR (PECOMA)
19.3.2 LYMPHANGIOLEIOMYOMATOSIS
19.3.3 OTHERS
19.4 SIROLIMUS CATHETER DEVICE
19.5 SIROLIMUS COATED BALLOONS
19.6 FACIAL ANGIOFIBROMA IN TUBEROUS SCLEROSIS COMPLEX (TSC)
19.7 OTHERS
20 GLOBAL SIROLIMUS MARKET , BY AGE GROUP
20.1 OVERVIEW
20.2 ADULT
20.3 PEDIATRIC
20.4 GERIARTIC
21 GLOBAL SIROLIMUS MARKET , BY GENDER
21.1 OVERVIEW
21.2 MALE
21.3 FEMALE
22 GLOBAL SIROLIMUS MARKET , BY END USER
22.1 OVERVIEW
22.2 HOSPITALS
22.2.1 PUBLIC
22.2.1.1. TIER I
22.2.1.2. TIER II
22.2.1.3. TIER III
22.2.2 PRIVATE
22.2.2.1. TIER I
22.2.2.2. TIER II
22.2.2.3. TIER III
22.3 SPECIALITY CLINICS
22.4 HOMECARE SETTING
22.5 AMBULATORY CARE CENTERS
22.6 CATH LABS
22.7 OTHERS
23 GLOBAL SIROLIMUS MARKET , BY DISTRIBUTION CHANNEL
23.1 OVERVIEW
23.2 DIRECT TENDER
23.3 RETAIL SALES
23.3.1 HOSPITAL PHARMACY
23.3.2 DRUG STORES
23.3.3 E-PHARMACY
23.3.4 OTHERS
23.4 OTHERS
24 GLOBAL SIROLIMUS MARKET , COMPANY LANDSCAPE
24.1 COMPANY SHARE ANALYSIS: GLOBAL
24.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
24.3 COMPANY SHARE ANALYSIS: EUROPE
24.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
24.5 MERGERS & ACQUISITIONS
24.6 NEW PRODUCT DEVELOPMENT & APPROVALS
24.7 EXPANSIONS
24.8 REGULATORY CHANGES
24.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
25 GLOBAL SIROLIMUS MARKET , BY REGION
GLOBAL SIROLIMUS MARKET , (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
25.1 NORTH AMERICA
25.1.1 U.S.
25.1.2 CANADA
25.1.3 MEXICO
25.2 EUROPE
25.2.1 GERMANY
25.2.2 U.K.
25.2.3 ITALY
25.2.4 FRANCE
25.2.5 SPAIN
25.2.6 RUSSIA
25.2.7 SWITZERLAND
25.2.8 TURKEY
25.2.9 BELGIUM
25.2.10 NETHERLANDS
25.2.11 DENMARK
25.2.12 SWEDEN
25.2.13 POLAND
25.2.14 NORWAY
25.2.15 FINLAND
25.2.16 REST OF EUROPE
25.3 ASIA-PACIFIC
25.3.1 JAPAN
25.3.2 CHINA
25.3.3 SOUTH KOREA
25.3.4 INDIA
25.3.5 SINGAPORE
25.3.6 THAILAND
25.3.7 INDONESIA
25.3.8 MALAYSIA
25.3.9 PHILIPPINES
25.3.10 AUSTRALIA
25.3.11 NEW ZEALAND
25.3.12 VIETNAM
25.3.13 TAIWAN
25.3.14 REST OF ASIA-PACIFIC
25.4 SOUTH AMERICA
25.4.1 BRAZIL
25.4.2 ARGENTINA
25.4.3 REST OF SOUTH AMERICA
25.5 MIDDLE EAST AND AFRICA
25.5.1 SOUTH AFRICA
25.5.2 EGYPT
25.5.3 BAHRAIN
25.5.4 UNITED ARAB EMIRATES
25.5.5 KUWAIT
25.5.6 OMAN
25.5.7 QATAR
25.5.8 SAUDI ARABIA
25.5.9 REST OF MEA
25.6 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
26 GLOBAL SIROLIMUS MARKET , COMPANY PROFILE
26.1 PFIZER INC.
26.1.1 COMPANY OVERVIEW
26.1.2 REVENUE ANALYSIS
26.1.3 GEOGRAPHIC PRESENCE
26.1.4 PRODUCT PORTFOLIO
26.1.5 RECENT DEVELOPMENTS
26.2 ZYDUS
26.2.1 COMPANY OVERVIEW
26.2.2 REVENUE ANALYSIS
26.2.3 GEOGRAPHIC PRESENCE
26.2.4 PRODUCT PORTFOLIO
26.2.5 RECENT DEVELOPMENTS
26.3 NOBELPHARMA AMERICA, LLC
26.3.1 COMPANY OVERVIEW
26.3.2 REVENUE ANALYSIS
26.3.3 GEOGRAPHIC PRESENCE
26.3.4 PRODUCT PORTFOLIO
26.3.5 RECENT DEVELOPMENTS
26.4 AADI BIOSCIENCE, INC.
26.4.1 COMPANY OVERVIEW
26.4.2 REVENUE ANALYSIS
26.4.3 GEOGRAPHIC PRESENCE
26.4.4 PRODUCT PORTFOLIO
26.4.5 RECENT DEVELOPMENTS
26.5 AMNEAL PHARMACEUTICALS NY LLC
26.5.1 COMPANY OVERVIEW
26.5.2 REVENUE ANALYSIS
26.5.3 GEOGRAPHIC PRESENCE
26.5.4 PRODUCT PORTFOLIO
26.5.5 RECENT DEVELOPMENTS
26.6 APOTEX CORP
26.6.1 COMPANY OVERVIEW
26.6.2 REVENUE ANALYSIS
26.6.3 GEOGRAPHIC PRESENCE
26.6.4 PRODUCT PORTFOLIO
26.6.5 RECENT DEVELOPMENTS
26.7 DR. REDDY'S LABORATORIES LTD.
26.7.1 COMPANY OVERVIEW
26.7.2 REVENUE ANALYSIS
26.7.3 GEOGRAPHIC PRESENCE
26.7.4 PRODUCT PORTFOLIO
26.7.5 RECENT DEVELOPMENTS
26.8 TORRENT PHARMACEUTICALS LTD.
26.8.1 COMPANY OVERVIEW
26.8.2 REVENUE ANALYSIS
26.8.3 GEOGRAPHIC PRESENCE
26.8.4 PRODUCT PORTFOLIO
26.8.5 RECENT DEVELOPMENTS
26.9 BIOCON
26.9.1 COMPANY OVERVIEW
26.9.2 REVENUE ANALYSIS
26.9.3 GEOGRAPHIC PRESENCE
26.9.4 PRODUCT PORTFOLIO
26.9.5 RECENT DEVELOPMENTS
26.1 INTAS PHARMACEUTICALS LTD
26.10.1 COMPANY OVERVIEW
26.10.2 REVENUE ANALYSIS
26.10.3 GEOGRAPHIC PRESENCE
26.10.4 PRODUCT PORTFOLIO
26.10.5 RECENT DEVELOPMENTS
26.11 CONCORD BIOTECH
26.11.1 COMPANY OVERVIEW
26.11.2 REVENUE ANALYSIS
26.11.3 GEOGRAPHIC PRESENCE
26.11.4 PRODUCT PORTFOLIO
26.11.5 RECENT DEVELOPMENTS
26.12 VIATRIS INC.
26.12.1 COMPANY OVERVIEW
26.12.2 REVENUE ANALYSIS
26.12.3 GEOGRAPHIC PRESENCE
26.12.4 PRODUCT PORTFOLIO
26.12.5 RECENT DEVELOPMENTS
26.13 NOVADOZ PHARMACEUTICALS.
26.13.1 COMPANY OVERVIEW
26.13.2 REVENUE ANALYSIS
26.13.3 GEOGRAPHIC PRESENCE
26.13.4 PRODUCT PORTFOLIO
26.13.5 RECENT DEVELOPMENTS
26.14 PHARMACEUTICAL ASSOCIATES, INC.
26.14.1 COMPANY OVERVIEW
26.14.2 REVENUE ANALYSIS
26.14.3 GEOGRAPHIC PRESENCE
26.14.4 PRODUCT PORTFOLIO
26.14.5 RECENT DEVELOPMENTS
26.15 ALKEM LABORATORIES LTD.,
26.15.1 COMPANY OVERVIEW
26.15.2 REVENUE ANALYSIS
26.15.3 GEOGRAPHIC PRESENCE
26.15.4 PRODUCT PORTFOLIO
26.15.5 RECENT DEVELOPMENTS
26.16 NOVITIUM PHARMA LLC
26.16.1 COMPANY OVERVIEW
26.16.2 REVENUE ANALYSIS
26.16.3 GEOGRAPHIC PRESENCE
26.16.4 PRODUCT PORTFOLIO
26.16.5 RECENT DEVELOPMENTS
26.17 AMNEAL PHARMACEUTICALS, INC.
26.17.1 COMPANY OVERVIEW
26.17.2 REVENUE ANALYSIS
26.17.3 GEOGRAPHIC PRESENCE
26.17.4 PRODUCT PORTFOLIO
26.17.5 RECENT DEVELOPMENTS
26.18 ACTIZA PHARMACEUTICAL PRIVATE LIMITED
26.18.1 COMPANY OVERVIEW
26.18.2 REVENUE ANALYSIS
26.18.3 GEOGRAPHIC PRESENCE
26.18.4 PRODUCT PORTFOLIO
26.18.5 RECENT DEVELOPMENTS
26.19 MSN LABORATORIES PRIVATE LIMITED
26.19.1 COMPANY OVERVIEW
26.19.2 REVENUE ANALYSIS
26.19.3 GEOGRAPHIC PRESENCE
26.19.4 PRODUCT PORTFOLIO
26.19.5 RECENT DEVELOPMENTS
26.2 GLENMARK PHARMS LTD
26.20.1 COMPANY OVERVIEW
26.20.2 REVENUE ANALYSIS
26.20.3 GEOGRAPHIC PRESENCE
26.20.4 PRODUCT PORTFOLIO
26.20.5 RECENT DEVELOPMENTS
26.21 MIDAS PHARMA GMBH
26.21.1 COMPANY OVERVIEW
26.21.2 REVENUE ANALYSIS
26.21.3 GEOGRAPHIC PRESENCE
26.21.4 PRODUCT PORTFOLIO
26.21.5 RECENT DEVELOPMENTS
26.22 TAJ PHARMA GROUP
26.22.1 COMPANY OVERVIEW
26.22.2 REVENUE ANALYSIS
26.22.3 GEOGRAPHIC PRESENCE
26.22.4 PRODUCT PORTFOLIO
26.22.5 RECENT DEVELOPMENTS
26.23 IVASCULAR S.L.U
26.23.1 COMPANY OVERVIEW
26.23.2 REVENUE ANALYSIS
26.23.3 GEOGRAPHIC PRESENCE
26.23.4 PRODUCT PORTFOLIO
26.23.5 RECENT DEVELOPMENTS
26.24 ALVIMEDICA
26.24.1 COMPANY OVERVIEW
26.24.2 REVENUE ANALYSIS
26.24.3 GEOGRAPHIC PRESENCE
26.24.4 PRODUCT PORTFOLIO
26.24.5 RECENT DEVELOPMENTS
26.25 CORDIS
26.25.1 COMPANY OVERVIEW
26.25.2 REVENUE ANALYSIS
26.25.3 GEOGRAPHIC PRESENCE
26.25.4 PRODUCT PORTFOLIO
26.25.5 RECENT DEVELOPMENTS
26.26 CONCEPT MEDICAL
26.26.1 COMPANY OVERVIEW
26.26.2 REVENUE ANALYSIS
26.26.3 GEOGRAPHIC PRESENCE
26.26.4 PRODUCT PORTFOLIO
26.26.5 RECENT DEVELOPMENTS
27 RELATED REPORTS
28 CONCLUSION
29 QUESTIONNAIRE
30 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.