Global Smallpox Treatment Market
Market Size in USD Million
CAGR :
% 
 USD
66.80 Million
USD
78.27 Million
2024
2032
USD
66.80 Million
USD
78.27 Million
2024
2032
| 2025 –2032 | |
| USD 66.80 Million | |
| USD 78.27 Million | |
|
|
|
|
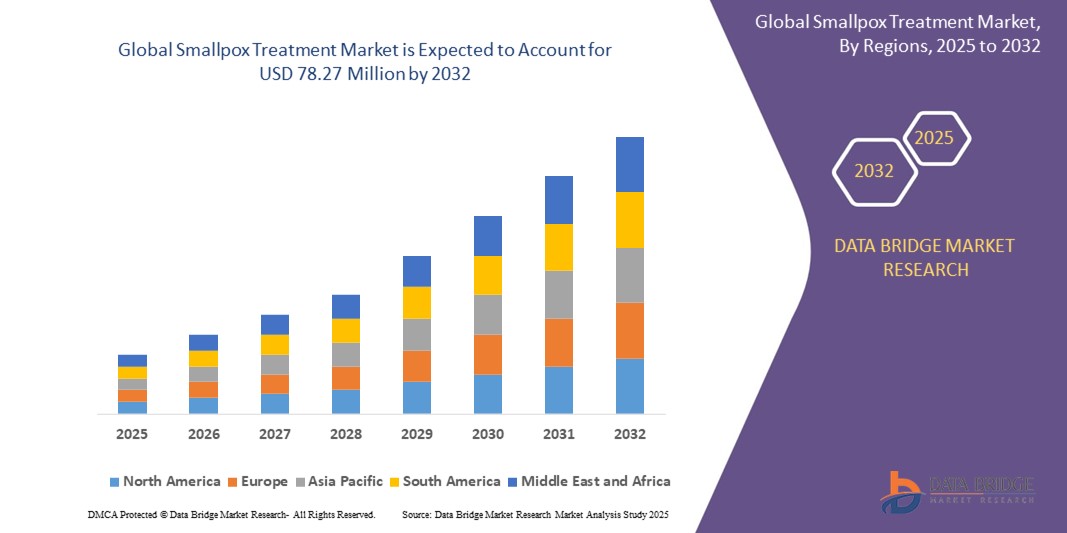
Smallpox Treatment Market Size
- The Global Smallpox Treatment Market size was valued at USD 66.8 million in 2024 and is expected to reach USD 78.27 million by 2032, at a CAGR of 2% during the forecast period
- The market growth is primarily driven by continued government investments in bioterrorism preparedness and the stockpiling of antiviral drugs and vaccines as part of public health security measures
- Additionally, advancements in antiviral therapies, growing research in orthopoxvirus countermeasures, and a focus on pandemic readiness are contributing to sustained demand for smallpox treatment options. These factors are ensuring a stable yet essential role for smallpox therapeutics in global infectious disease preparedness, supporting modest but consistent market expansion
Smallpox Treatment Market Analysis
- Smallpox treatment comprises antiviral medications and vaccines aimed at managing outbreaks of Variola virus infections, with a focus on emergency preparedness, bioterrorism defense, and post-eradication containment strategies. These treatments are essential for public health security programs worldwide, especially as the threat of orthopoxvirus-related diseases resurfaces
- The rising demand for smallpox treatment is primarily fueled by increasing global investments in pandemic preparedness, expanding government stockpiling of antiviral agents such as Tecovirimat, and ongoing advancements in next-generation vaccine development. The emphasis on rapid response mechanisms and infection containment is also accelerating the adoption of treatment protocols across various healthcare systems
- North America dominates the smallpox treatment market with the largest revenue share of 47.6% in 2025, owing to its well-established healthcare infrastructure, significant government procurement programs for strategic national stockpiles, and active involvement of key pharmaceutical players. The U.S. leads in terms of R&D funding, emergency use authorizations, and military-grade medical preparedness
- The Asia-Pacific region is projected to be the fastest-growing market for smallpox treatment during the forecast period, driven by increased awareness about infectious disease threats, rising healthcare expenditures, and stronger public health collaborations with international agencies
- The vaccination segment is expected to dominate the smallpox treatment market with a market share of 58.3% in 2025, supported by the historical success of vaccines like ACAM2000 and growing efforts to develop safer, next-generation formulations such as MVA-BN (JYNNEOS)
Report Scope and Smallpox Treatment Market Segmentation
|
Attributes |
Smallpox Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Smallpox Treatment Market Trends
“Next-Generation Therapeutics and Vaccine Innovation in Biodefense”
- A major trend shaping the global smallpox treatment market is the advancement of next-generation vaccines and antiviral drugs aimed at strengthening biodefense and global pandemic preparedness. Following the eradication of smallpox, the disease remains a high-priority biosecurity threat, prompting governments and organizations to invest in improved countermeasures
- For instance, SIGA Technologies’ Tecovirimat (TPOXX) is the first antiviral approved specifically for the treatment of smallpox, marking a shift toward targeted pharmaceutical preparedness. Similarly, Bavarian Nordic’s MVA-BN (marketed as JYNNEOS) has gained prominence as a non-replicating vaccine for both smallpox and monkeypox, increasingly favored due to its improved safety profile compared to legacy vaccines like ACAM2000
- Recent innovations are also focusing on temperature-stable formulations, intradermal administration, and dual-action antivirals to improve logistics and efficacy in emergency use scenarios. Companies like Tonix Pharmaceuticals and Chimerix are exploring alternative delivery platforms and novel compounds to expand the therapeutic landscape for orthopoxvirus infections
- The increasing collaboration between biotech firms and federal agencies such as the U.S. Biomedical Advanced Research and Development Authority (BARDA) is further accelerating product development and stockpiling contracts. These collaborations not only support innovation but also ensure readiness for rapid deployment in the event of an outbreak or bioterrorist threat
- This trend of investing in intelligent, accessible, and high-efficacy smallpox countermeasures is reshaping the market’s trajectory from passive storage toward dynamic, responsive biodefense capabilities. It reflects growing governmental and institutional awareness of emerging zoonotic threats and the importance of updated medical countermeasures
Smallpox Treatment Market Dynamics
Driver
“Government Funding and Strategic Stockpiling for Bioterrorism Preparedness”
- A primary driver of the global smallpox treatment market is the intensifying government focus on biodefense and pandemic response preparedness. Agencies such as BARDA (U.S.), Public Health England, and the European Centre for Disease Prevention and Control (ECDC) are expanding strategic national stockpiles of vaccines and antivirals to mitigate the risks posed by smallpox and related orthopoxviruses
- For instance, Emergent BioSolutions and SIGA Technologies have both secured multi-year supply contracts with the U.S. government for smallpox vaccines and therapeutics under Project BioShield and the Strategic National Stockpile initiative. These partnerships not only provide consistent revenue streams but also underscore the importance of long-term preparedness
- The rising global concern about bioterrorism and zoonotic disease resurgence, especially following monkeypox outbreaks, is reinforcing the need for rapid access to safe and effective smallpox countermeasures. This awareness is fueling global demand, particularly in countries seeking to bolster their infectious disease containment capacity
- In parallel, increased clinical investments, streamlined regulatory pathways for emergency use authorization, and public-private partnerships are creating a favorable environment for sustained market growth
Restraint/Challenge
“Limited Commercial Market and High R&D Costs”
- Despite its strategic importance, the smallpox treatment market is constrained by the absence of naturally occurring cases, making it heavily reliant on government procurement and emergency preparedness budgets. This limits commercial viability and discourages some pharmaceutical companies from pursuing product development in the absence of guaranteed contracts
- The high cost and complexity of developing antiviral drugs and next-generation vaccines, including the need for long-term safety and efficacy studies, pose significant challenges to market entry. Companies must often invest heavily in research, preclinical development, and facility compliance with Good Manufacturing Practices (GMP), with few opportunities for traditional commercial returns
- Moreover, regulatory hurdles related to biodefense products—including dual-use concerns and ethical challenges around human testing for eradicated diseases—further complicate market access and delay product launches
- Another challenge lies in the limited awareness and preparedness in low- and middle-income countries, where budget constraints, competing health priorities, and lack of infrastructure may impede the widespread deployment of smallpox countermeasures
- Overcoming these challenges requires global alignment on preparedness priorities, greater international funding mechanisms, and continued collaboration between governments and biotech innovators to ensure that smallpox therapeutics remain a viable and scalable element of global health security.
Smallpox Treatment Market Scope
The market is segmented on the basis of type, treatment type, route of administration, end users, and distribution channel.
- By Type
On the basis of type, the smallpox treatment market is segmented into Ordinary Smallpox (Variola Major), Sequelae, Modified-Type Smallpox, and Others. The Ordinary Smallpox (Variola Major) segment dominates the market with the largest revenue share of 62.4% in 2025, as it represents the most common and historically severe form of the disease. Governments and health agencies prioritize treatments and vaccines targeted at this type for strategic stockpiling due to its high transmissibility and mortality rate. Its dominance is reinforced by historical data, modeling, and the focus of most therapeutic pipelines.
The Sequelae segment is expected to witness the fastest compound annual growth rate (CAGR) of 3.6% from 2025 to 2032, as post-infection complications such as scarring, blindness, and joint deformities carry long-term healthcare burdens. The rising interest in rehabilitation therapies, dermatological interventions, and supportive care for survivors of orthopoxvirus infections is driving growth in this segment.
• By Treatment Type
On the basis of treatment type, the market is segmented into Medication, Vaccination, and Others. The Vaccination segment holds the largest market revenue share in 2025, driven by its historical success in eradicating smallpox and its central role in current biosecurity strategies. Products like ACAM2000 and MVA-BN (JYNNEOS) are widely used in preparedness programs and emergency response protocols.
The Medication segment is projected to grow at the fastest CAGR during the forecast period, supported by increased approval and use of antiviral agents like Tecovirimat (TPOXX) and Brincidofovir, especially for treatment in cases of exposure or complications.
• By Route of Administration
On the basis of route of administration, the smallpox treatment market is segmented into Oral, Injectable, and Others. The Injectable segment accounted for the largest market share in 2025, due to its widespread use for vaccine delivery and the high efficacy of injectable antiviral formulations in emergency treatment protocols.
The Oral segment is expected to experience the fastest growth from 2025 to 2032, driven by the development of oral antivirals like Tecovirimat, which offer ease of administration, especially in mass treatment scenarios and for home-based care during outbreaks.
• By End Users
On the basis of end users, the market is segmented into Hospitals, Homecare, Specialty Clinics, and Others. Hospitals dominated the market in 2025 with the highest revenue share, as they serve as the primary centers for patient diagnosis, emergency treatment, and administration of vaccines under controlled clinical settings during outbreaks or public health emergencies.
The Homecare segment is projected to witness the fastest CAGR during the forecast period, as governments and health systems increasingly focus on decentralized response strategies and at-home treatment options using oral antivirals and self-administered vaccines to manage exposure risks during potential outbreaks.
• By Distribution Channel
On the basis of distribution channel, the smallpox treatment market is segmented into Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, and Others. Hospital Pharmacy holds the largest revenue share in 2025, due to its role in handling emergency stockpiles and coordinating closely with public health agencies during outbreak response.
The Online Pharmacy segment is expected to grow at the fastest pace from 2025 to 2032, due to increased digitalization of healthcare, government authorization for controlled medication delivery, and the need for rapid deployment of treatments in remote or quarantine settings.
Smallpox Treatment Market Regional Analysis
- North America dominates the global smallpox treatment market with the largest revenue share of 47.6% in 2024, driven by strong government-led preparedness programs, well-established healthcare infrastructure, and significant investment in strategic national stockpiles of vaccines and antiviral drugs.
- The region’s dominance is reinforced by the presence of leading pharmaceutical companies and biotech firms engaged in smallpox drug and vaccine development, such as SIGA Technologies and Emergent BioSolutions.
- High levels of public health awareness, ongoing federal funding for biodefense, and advanced capabilities for infectious disease management position North America as the key hub for smallpox preparedness, with widespread adoption of next-generation solutions in both civil and military sectors.
U.S. Smallpox Treatment Market Insight
The U.S. smallpox treatment market captured the largest revenue share of 83% within North America in 2025, supported by significant federal funding for biodefense, advanced R&D capabilities, and strong regulatory infrastructure. The U.S. Strategic National Stockpile continues to invest in vaccines like ACAM2000 and MVA-BN (JYNNEOS), and antiviral treatments such as Tecovirimat (TPOXX). The country’s proactive approach to bioterrorism preparedness, along with public-private partnerships with companies like SIGA Technologies and Emergent BioSolutions, plays a critical role in maintaining leadership in this space.
Europe Smallpox Treatment Market Insight
The European smallpox treatment market is projected to expand at a steady CAGR during the forecast period, driven by increasing public health preparedness initiatives and cross-border collaborations under the EU Health Security Committee. The region has been boosting its stockpiling strategies and surveillance systems to combat the potential resurgence of orthopoxviruses, especially in light of recent monkeypox outbreaks. European regulatory bodies are also supporting accelerated approval pathways for emergency-use vaccines and antivirals.
U.K. Smallpox Treatment Market Insight
The U.K. smallpox treatment market is expected to grow at a moderate CAGR, driven by growing investment in national biosecurity infrastructure and infectious disease response capabilities. Public Health England and NHS initiatives are expanding readiness through the acquisition of approved smallpox countermeasures. The U.K.'s focus on next-generation vaccine safety and access, combined with partnerships with biotech firms, is reinforcing its position as a key European player in pandemic preparedness.
Germany Smallpox Treatment Market Insight
The German smallpox treatment market is anticipated to grow at a consistent pace, supported by strong healthcare infrastructure, federal government preparedness strategies, and funding for infectious disease research. Germany’s emphasis on data-driven public health planning and its integration with EU-wide disease control mechanisms enable coordinated stockpiling and vaccine distribution. Biotech innovation in antiviral R&D is also on the rise, contributing to local and regional resilience.
Asia-Pacific Smallpox Treatment Market Insight
The Asia-Pacific smallpox treatment market is poised to grow at the fastest CAGR of over 4.1% in 2025, driven by increasing investments in pandemic preparedness, population health management, and regional public health capacity-building. Countries such as China, Japan, South Korea, and India are strengthening biosecurity frameworks and collaborating with international bodies like WHO to secure vaccine access and emergency therapeutics. The growing awareness of zoonotic virus threats, following monkeypox outbreaks, is accelerating market development across both public and private healthcare sectors.
Japan Smallpox Treatment Market Insight
The Japan smallpox treatment market is gaining traction, supported by the country's emphasis on public health innovation, disaster preparedness, and bioterrorism defense. Japan’s well-organized healthcare infrastructure and regulatory efficiency enable rapid procurement and distribution of antivirals and vaccines. Government funding for research on orthopoxvirus countermeasures, combined with public concern over infectious disease threats, is stimulating market growth.
China Smallpox Treatment Market Insight
The China smallpox treatment market accounted for the largest revenue share in Asia-Pacific in 2025, attributed to robust government-led disease surveillance, domestic vaccine production capacity, and rapid deployment capabilities. China’s National Health Commission is investing heavily in emergency response systems and mass vaccination strategies. Additionally, the country’s expanding biotech ecosystem and its emphasis on self-sufficiency in critical health technologies are bolstering domestic development and availability of smallpox treatment solutions.
Smallpox Treatment Market Share
The Smallpox Treatment industry is primarily led by well-established companies, including:
- SIGA Technologies (U.S.)
- Bavarian Nordic (Denmark)
- EpiVax, Inc. (U.S.)
- CEL-SCI Corporation (U.S.)
- Chimerix (U.S.)
- Nano Therapeutics Pvt Ltd (India)
- Oncovir, Inc. (U.S.)
- Symphogen (Denmark)
- Marker Therapeutics, Inc. (U.S.)
- Tonix Pharmaceuticals Holding Corp. (U.S.)
- Sanofi (France)
- Emergent BioSolutions Inc. (U.S.)
- General Dynamics Information Technology, Inc. (U.S.)
- Pfizer Inc. (U.S.)
- GlaxoSmithKline plc (GSK) (U.K.)
Latest Developments in Global Smallpox Treatment Market
- In March 2025, Bavarian Nordic A/S (OMX: BAVA) announced that the U.S. Food and Drug Administration (FDA) has approved the freeze-dried formulation of JYNNEOS® (Smallpox and Mpox Vaccine, Live, Non-replicating) for the prevention of smallpox and mpox in adults 18 years and older. This approval enhances flexibility for stockpiling in response to smallpox or mpox outbreaks.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL SMALLPOX TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL SMALLPOX TREATMENT MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY BASED MODEL
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL SMALLPOX TREATMENT MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4 EXECUTIVE SUMMARY
5 PREMIUM INSIGHTS
5.1 CONVENTIONAL VACCINE – DRYVAX
5.2 OTHERS
6 EPIDEMIOLOGY
7 INDUSTRY INSIGHTS
8 REGULATORY FRAMEWORK
9 PIPELINE ANALYSIS
9.1 OVERVIEW
9.2 PHASE III CANDIDATES
9.3 PHASE II CANDIDATES
9.4 PHASE I CANDIDATES
9.5 OTHERS
10 IMPACT OF COVID-19 PANDEMIC ON THE MARKET
10.1 PRICE IMPACT
10.2 IMPACT ON DEMAND
10.3 IMPACT ON SUPPLY CHAIN
10.4 STRATEGIC DECISIONS FOR MANUFACTURERS
10.5 CONCLUSION
11 GLOBAL SMALLPOX TREATMENT MARKET, BY TREATMENT
11.1 OVERVIEW
11.2 VACCINE(PREVENTION)
11.2.1 CONVENTIONAL VACCINE
11.2.1.1. APSV
11.2.1.2. OTHERS
11.2.2 TISSUE CULTURE BASED, LIVE VIRUS VACCINES
11.2.2.1. ACAM1000
11.2.2.2. ACAM2000
11.2.2.3. OTHERS
11.2.3 REPLICATION COMPETENT, ATTENUATED VACCINES
11.2.3.1. MVA-BN
11.2.3.2. VACCINIA
11.2.3.3. NYVAC
11.2.3.4. IMVAMUNE
11.2.4 SUBUNIT VACCINES
11.2.4.1. PROTEIN
11.2.4.2. DNA
11.2.4.3. OTHERS
11.3 ANTIVIRAL DRUGS (CURE)
11.3.1.1. TECOVIRIMAT
11.3.1.2. BRINCIDOFOVIR
11.3.1.3. CIDOFOVIR
11.3.1.4. OTHERS
12 GLOBAL SMALLPOX TREATMENT MARKET, BY PRODUCT TYPE
12.1.1 OVERVIEW
12.1.2 MONOVALENT
12.1.3 MULTIVALENT
13 GLOBAL SMALLPOX TREATMENT MARKET, BY STRAIN TYPE
13.1 OVERVIEW
13.2 NYCBOH
13.3 LISTER
13.4 ANKARA
13.5 COPENHAGEN
13.6 MVA
13.7 VACV
13.8 VARV
14 GLOBAL SMALLPOX TREATMENT MARKET, BY AGE GROUP
14.1 OVERVIEW
14.2 PAEDIATRIC
14.3 ADULTS
14.4 GERIATRICS
15 GLOBAL SMALLPOX TREATMENT MARKET, BY ROUTE OF ADMINISTRATION
15.1 OVERVIEW
15.2 ORAL
15.3 INJECTABLES
15.3.1 SC
15.3.2 IM
15.3.3 IC
15.3.4 IP
15.4 OTHERS
16 GLOBAL SMALLPOX TREATMENT MARKET, BY END USER
16.1 OVERVIEW
16.2 HOSPITALS
16.2.1 GOVERNMENT
16.2.2 PRIVATE
16.3 HOMECARE
16.4 SPECIALTY CLINICS
16.5 OTHERS.
17 GLOBAL SMALLPOX TREATMENT MARKET, BY DISTRIBUTION CHANNEL
17.1 OVERVIEW
17.2 DIRECT TENDER
17.3 RETAIL SALES
17.3.1 ONLINE STORES
17.3.2 PHARMACY STORES
17.3.3 OTHERS
17.4 OTHERS
18 GLOBAL SMALLPOX TREATMENT MARKET, BY GEOGRAPHY
Global Smallpox treatment market, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
18.1.1 NORTH AMERICA
18.1.1.1. U.S.
18.1.1.2. CANADA
18.1.1.3. MEXICO
18.1.2 EUROPE
18.1.2.1. GERMANY
18.1.2.2. FRANCE
18.1.2.3. U.K.
18.1.2.4. HUNGARY
18.1.2.5. LITHUANIA
18.1.2.6. AUSTRIA
18.1.2.7. IRELAND
18.1.2.8. NORWAY
18.1.2.9. POLAND
18.1.2.10. ITALY
18.1.2.11. SPAIN
18.1.2.12. RUSSIA
18.1.2.13. TURKEY
18.1.2.14. BELGIUM
18.1.2.15. NETHERLANDS
18.1.2.16. SWITZERLAND
18.1.2.17. REST OF EUROPE
18.1.3 ASIA-PACIFIC
18.1.3.1. JAPAN
18.1.3.2. CHINA
18.1.3.3. SOUTH KOREA
18.1.3.4. BANGLADESH
18.1.3.5. AUSTRALIA
18.1.3.6. SINGAPORE
18.1.3.7. THAILAND
18.1.3.8. MALAYSIA
18.1.3.9. INDONESIA
18.1.3.10. PHILIPPINES
18.1.3.11. VIETNAM
18.1.3.12. REST OF ASIA-PACIFIC
18.1.4 SOUTH AMERICA
18.1.4.1. BRAZIL
18.1.4.2. ARGENTINA
18.1.4.3. PERU
18.1.4.4. REST OF SOUTH AMERICA
18.1.5 MIDDLE EAST AND AFRICA
18.1.5.1. SOUTH AFRICA
18.1.5.2. SAUDI ARABIA
18.1.5.3. UAE
18.1.5.4. EGYPT
18.1.5.5. KUWAIT
18.1.5.6. ISRAEL
18.1.5.7. REST OF MIDDLE EAST AND AFRICA
19 GLOBAL SMALLPOX TREATMENT MARKET, COMPANY LANDSCAPE
19.1 COMPANY SHARE ANALYSIS: GLOBAL
19.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
19.3 COMPANY SHARE ANALYSIS: EUROPE
19.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
19.5 MERGERS & ACQUISITIONS
19.6 NEW PRODUCT DEVELOPMENT & APPROVALS
19.7 EXPANSIONS
19.8 REGULATORY CHANGES
19.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
20 GLOBAL SMALLPOX TREATMENT MARKET, SWOT AND DBMR ANALYSIS
21 GLOBAL SMALLPOX TREATMENT MARKET, COMPANY PROFILE
21.1 BAVARIAN NORDIC
21.1.1 COMPANY OVERVIEW
21.1.2 REVENUE ANALYSIS
21.1.3 GEOGRAPHIC PRESENCE
21.1.4 PRODUCT PORTFOLIO
21.1.5 RECENT DEVELOPMENTS
21.2 EMERGENT BIOSOLUTIONS INC
21.2.1 COMPANY OVERVIEW
21.2.2 REVENUE ANALYSIS
21.2.3 GEOGRAPHIC PRESENCE
21.2.4 PRODUCT PORTFOLIO
21.2.5 RECENT DEVELOPMENTS
21.3 SIGA TECHNOLOGIES
21.3.1 COMPANY OVERVIEW
21.3.2 REVENUE ANALYSIS
21.3.3 GEOGRAPHIC PRESENCE
21.3.4 PRODUCT PORTFOLIO
21.3.5 RECENT DEVELOPMENTS
21.4 CHIMERIX
21.4.1 COMPANY OVERVIEW
21.4.2 REVENUE ANALYSIS
21.4.3 GEOGRAPHIC PRESENCE
21.4.4 PRODUCT PORTFOLIO
21.4.5 RECENT DEVELOPMENTS
21.5 GILEAD SCIENCES
21.5.1 COMPANY OVERVIEW
21.5.2 REVENUE ANALYSIS
21.5.3 GEOGRAPHIC PRESENCE
21.5.4 PRODUCT PORTFOLIO
21.5.5 RECENT DEVELOPMENTS
21.6 BIOFACTURA, INC.
21.6.1 COMPANY OVERVIEW
21.6.2 REVENUE ANALYSIS
21.6.3 GEOGRAPHIC PRESENCE
21.6.4 PRODUCT PORTFOLIO
21.6.5 RECENT DEVELOPMENTS
21.7 TONIX PHARMACEUTICALS HOLDING CORP.
21.7.1 COMPANY OVERVIEW
21.7.2 REVENUE ANALYSIS
21.7.3 GEOGRAPHIC PRESENCE
21.7.4 PRODUCT PORTFOLIO
21.7.5 RECENT DEVELOPMENTS
*NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
22 RELATED REPORTS
23 CONCLUSION
24 QUESTIONNAIRE
25 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.