Global Stem Cell Therapy Market
Market Size in USD Million
CAGR :
% 
 USD
257.00 Million
USD
921.12 Million
2022
2030
USD
257.00 Million
USD
921.12 Million
2022
2030
| 2023 –2030 | |
| USD 257.00 Million | |
| USD 921.12 Million | |
|
|
|
|
Stem Cell Therapy Market Analysis and Size
The World Health Organization (WHO) estimates cerebrovascular diseases and neurological disorders account for around 7.1% of the global disease burden. As a result, businesses are carrying out fundamental research and preclinical studies to examine stem cells' ability to regenerate in treating neurological diseases. Cellular treatments for cancer are currently receiving significant financing from companies, which is expected to support market expansion.
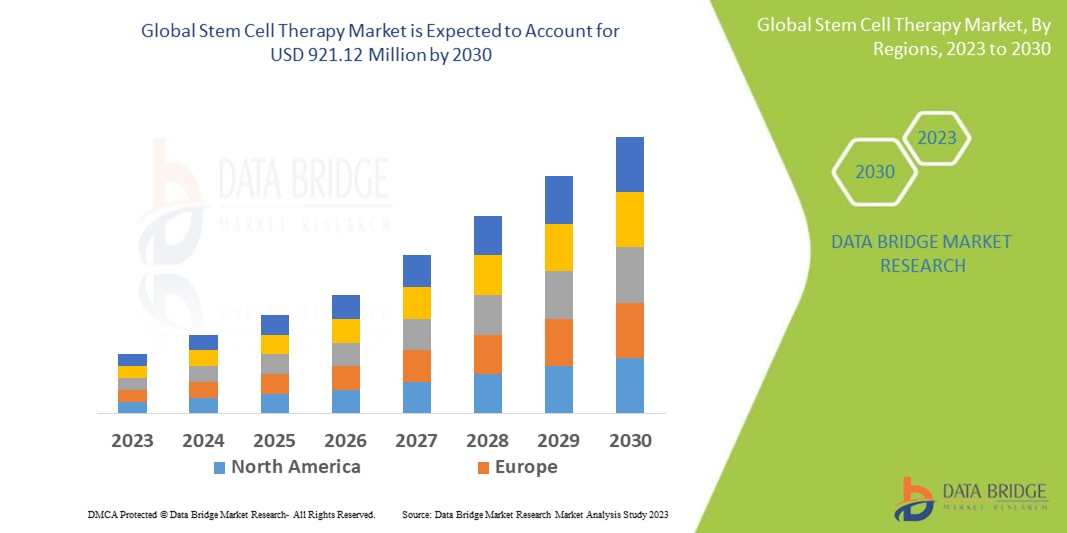
Data Bridge Market Research analyses that the stem cell therapy market, which is USD 257 million in 2022, is expected to reach USD 921.12 million by 2030, at a CAGR of 17.3% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Stem Cell Therapy Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product Type (Bone Marrow Derived Mesenchymal Cells, Placental or Umbilical Stem Cell, Adipose Tissue Derived Mesenchymal Stem Cells, and Others), Type (Allogenic Stem Cell Therapy and Autologous Stem Cell Therapy), Application (Musculoskeletal Disorders, Acute Graft-Versus-Host Disease (AGVHD), Wounds and Injuries, Cardiovascular Diseases, Surgeries, Gastrointestinal Diseases, and Others), End User (Hospitals and Surgical Centers, Therapeutic Companies, Services Companies, and Others), Distribution Channel (Direct Tender, Third Party Distributors) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
Athersys Inc. (U.S.), Mesoblast Ltd (Australia), Biorestorative Therapies Inc. (U.S.), Pluristem Inc. (Israel), Brainstorm Cell Limited. (U.S.), ViaCyte Inc. (U.S.), Gamida Cell (U.S.), HOPE BIOSCIENCES (U.S.), Cellular Biomedicine Group (U.S.), Smith+Nephew (U.K.), MEDIPOST (South Korea), ANTEROGEN. C.O., LTD. (South Korea), NuVasive Inc. (U.S.), RTI Surgical (U.S.), AlloSource (U.S.), JCR Pharmaceuticals Co. Ltd. (Japan), Takeda Pharmaceutical Company Limited (Japan) |
|
Market Opportunities |
|
Market Definition
Stem cell therapy is regenerative medicininal therapy used to repair damaged cells by lowering inflammation and controlling the immune system. This makes stem cell therapy an effective remedy for several illnesses. Studies on stem cell therapies for Crohn's disease, Multiple Sclerosis, Lupus, COPD, Parkinson's, ALS, stroke recovery, and more have been undertaken. Stem cell therapies have also been utilized to treat autoimmune, inflammatory, neurological, orthopedic, and traumatic disorders.
Stem Cell Therapy Market Dynamics
Drivers
- The rise in prevalence and incidence of chronic diseases will drive the market growth
The majority of people around the world suffer from chronic ailments. One in three adults worldwide has a chronic illness. Chronic diseases have impacted the health and quality of life of many people. Chronic illnesses, such as cancer, musculoskeletal and neurological conditions, chronic injuries, cardiovascular and gastrointestinal conditions, and cancer, can result in hospitalization, long-term incapacity, a decline in quality of life, and even death.
The mesenchymal stem cells penetrate and integrate into several organs, treat lung, spinal cord, autoimmune disorders, liver, bone, and cartilage diseases, and treat multiple organ damage. Using stem cells in the therapy of inflammatory, immune system, and degenerative tissue illnesses is an effective strategy.
- The growing need for potent treatments will propel the market growth
One of the key drivers of market expansion is significant R&D investments. In addition, the growing need for potent treatments to reduce disease burden during the forecast period is another factor fuelling the growth. For instance, the 5-year exploratory study on Parkinson's illness by Celavie Biosciences is still ongoing as of May 2020. For the treatment of Parkinson's disease and other illnesses of the central nervous system, the business is developing regenerative stem cell therapies. Ok99 stem cell-based exploratory clinical studies for Parkinson's disease were effective, according to Celavie Biosciences.
Opportunities
- An increase in R&D activities will act as an opportunity
Additionally, the increase in R&D activities and rising investments from public and private organizations will open up new possibilities for the market's growth rate. For instance, U.S. healthcare spending increased by 3.4% in 2021, per the Health Care Price Index (HCPI). The rise in growth indicates that federal spending fell sharply the year before, from USD 287,000 million in 2020 to USD 170,000 million in 2021.
The prompt treatment of chronic illnesses has increased the demand for stem cell therapy in the U.S. and Europe. Due to these positive elements, there is a greater need for drugs, and both major and minor market players are employing various techniques to meet this demand.
- A strategic initiative by market players will widen the scope of growth
The leading companies are also working to develop targeted strategies, including product launches, acquisitions, approvals, expansions, and partnerships, to ensure the smooth operation of the business, minimize risks, and boost the market's long-term growth in sales.
For instance,
To create and market the search-use-only (RUO) microfluidic intracellular delivery technology, ViaCyte, Inc. teamed up with SQZ Biotechnologies in May 2022. Through the agreement, both market participants will be able to share fresh cell engineering research in hematopoietic stem cells.
Restraints/Challenges
- The high cost of treatment hinders the market expansion
Cell treatment market expansion has been hampered by its high cost. As people search for the newest therapy choices, cell treatments have emerged as a popular treatment option. Cell treatments are still exceedingly expensive to try, despite the enormous rise in demand for them. Depending on the situation, simple joint injections might cost up to $1,000, and more complex operations can cost up to $100,000. In the U.S., the average price of stem cell therapy is projected to be between $4,000 and $8,000 per patient in 2020. Therefore, the growth of the stem cell therapy market is restrained by the high cost of the treatment.
This stem cell therapy market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the stem cell therapy market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Recent Developments
- In 2021, A worldwide licensed agreement and strategic collaboration between Minovia Therapeutics, Ltd. and Astellas Pharma, Inc. was established for the development of cutting-edge stem cell therapy programs for illnesses brought on by mitochondrial malfunction. The partnership intends to accelerate the development of allogeneic mitochondrial cell therapy initiatives. The two businesses will collaborate to investigate potential cell treatment program prospects using Astellas' genetically modified, induced pluripotent stem cells and Minovia's MAT platform technology.
Global Stem Cell Therapy Market Scope
The stem cell therapy market is segmented based on type, usage, end-user, and application. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product Type
- Bone Marrow Derived Mesenchymal Cells
- Placental or Umbilical Stem Cell
- Adipose Tissue Derived Mesenchymal Stem Cells
- Others
Type
- Allogenic Stem Cell Therapy
- Autologous Stem Cell Therapy
Application
- Musculoskeletal Disorders
- Acute Graft-Versus-Host Disease (AGVHD)
- Wounds and Injuries
- Cardiovascular Diseases
- Surgeries
- Gastrointestinal Diseases
- Others
End User
- Hospitals
- Surgical Centers
- Therapeutic Companies
- Services Companies
- Others
Distribution Channel
- Direct Tender
- Third Party Distributors
Stem Cell Therapy Market Regional Analysis/Insights
The stem cell therapy market is analysed and market size insights and trends are provided by country, type, usage, end-user and application as referenced above.
The countries covered in the stem cell therapy market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America dominates the stem cell therapy market because of the region's well-developed healthcare infrastructure and favourable reimbursement policies. Another factor contributing to the region's growth is the number of government initiatives to promote stem cell therapy.
Asia-Pacific is expected to grow at the highest growth rate in the forecast period of 2023 to 2030, owing to the growing incidence of cancer cases, rising technological advancements, and rising prevalence of chronic diseases such as diabetes, cancer, and neurological disorders.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Stem Cell Therapy Market Share Analysis
The stem cell therapy market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to stem cell therapy market.
Some of the major players operating in the stem cell therapy market are:
- Athersys Inc. (U.S.)
- Mesoblast Ltd (Australia)
- Biorestorative Therapies Inc. (U.S.)
- Pluristem Inc. (Israel)
- Brainstorm Cell Limited. (U.S.)
- ViaCyte Inc. (U.S.)
- Gamida Cell (U.S.)
- HOPE BIOSCIENCES (U.S.)
- Cellular Biomedicine Group (U.S.)
- Smith+Nephew (U.K.)
- MEDIPOST (South Korea)
- ANTEROGEN. C.O., LTD. (South Korea)
- NuVasive Inc. (U.S.)
- RTI Surgical (U.S.)
- AlloSource (U.S.)
- JCR Pharmaceuticals Co. Ltd. (Japan)
- Takeda Pharmaceutical Company Limited (Japan)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.












