Global Sterilization Monitoring Market
Market Size in USD Million
CAGR :
% 
 USD
731.74 Million
USD
1.32 Million
2024
2032
USD
731.74 Million
USD
1.32 Million
2024
2032
| 2025 –2032 | |
| USD 731.74 Million | |
| USD 1.32 Million | |
|
|
|
|
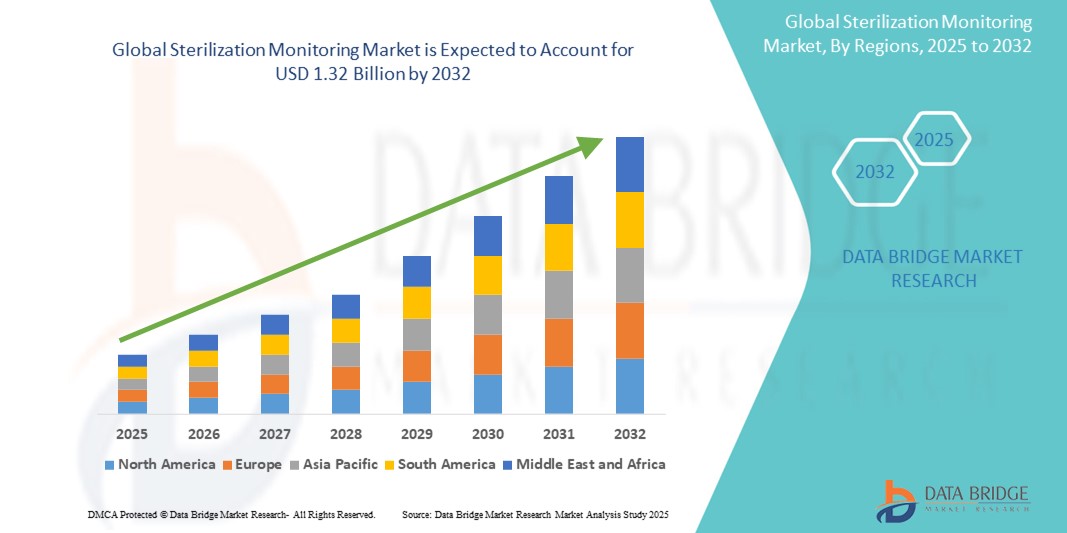
Sterilization Monitoring Market Size
- The global Sterilization Monitoring market was valued at USD 731.74 million in 2024 and is expected to reach USD 1.32 billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 7.61%, primarily driven by the increasing demand for infection control in healthcare settings and stringent regulatory requirements for sterilization procedures
- This growth is driven by factors such as the rising prevalence of hospital-acquired infections and the growing adoption of sterilization monitoring technologies across healthcare facilities
Sterilization Monitoring Market Analysis
- Sterilization monitoring systems are essential for ensuring the effectiveness of sterilization processes in medical, pharmaceutical, and laboratory settings. These systems validate that equipment and instruments are properly sterilized to prevent infections and maintain compliance with health regulations
- The market demand is largely driven by the rising incidence of hospital-acquired infections (HAIs), increased emphasis on infection control protocols, and stricter regulatory standards in healthcare facilities worldwide
- North America emerges as a leading region in this market, owing to its robust healthcare infrastructure, stringent safety standards, and widespread adoption of advanced sterilization technologies
- For instance, the regulatory bodies such as the CDC and FDA in the U.S. mandate regular sterilization monitoring, propelling consistent demand from hospitals, dental clinics, and ambulatory surgical center
- Globally, sterilization monitoring products are considered vital components in infection prevention strategies and are increasingly being integrated into automated sterilization workflows to enhance safety, efficiency, and compliance
Report Scope and Sterilization Monitoring Market Segmentation
|
Attributes |
Sterilization Monitoring Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Sterilization Monitoring Market Trends
“Shift Toward Automation and Smart Sterilization Monitoring Systems”
- One prominent trend in the global sterilization monitoring market is the growing shift toward automation and the use of smart monitoring systems integrated with digital technologies.
- These innovations streamline the sterilization process by reducing manual errors, ensuring real-time tracking, and enhancing compliance with regulatory standards through automated data logging and reporting
- For instance, smart sterilization indicators equipped with RFID or barcode scanning enable automatic cycle verification, immediate detection of sterilization failures, and improved traceability across healthcare and laboratory environments
- Automated systems also support integration with hospital information systems (HIS), allowing centralized monitoring and digital recordkeeping, which is crucial for audits and quality assurance
- This trend is transforming infection control practices by increasing efficiency, accountability, and safety, thereby driving the demand for technologically advanced sterilization monitoring solutions across the globe
Sterilization Monitoring Market Dynamics
Driver
“Rising Focus on Infection Prevention and Regulatory Compliance”
- The growing global emphasis on infection prevention, particularly in healthcare settings, is a major driver fueling the demand for sterilization monitoring products. With the rise in hospital-acquired infections (HAIs), ensuring effective sterilization has become a critical component of patient safety protocols
- Healthcare facilities are under increasing pressure to meet stringent guidelines set by regulatory bodies such as the CDC, FDA, and WHO, which mandate routine monitoring of sterilization processes to ensure compliance and patient protection
- Sterilization monitoring products, such as biological indicators, chemical indicators, and integrators, are essential in validating sterilization cycles and confirming the sterility of surgical instruments, laboratory tools, and reusable medical devices
- The expanding volume of surgical procedures, coupled with the need for validated sterile environments, further accelerates the adoption of reliable sterilization monitoring tools across hospitals, dental clinics, pharmaceutical companies, and diagnostic labs
- As infection control remains a cornerstone of quality healthcare delivery, the sterilization monitoring market is seeing increased investments and innovations aimed at improving reliability and efficiency
For instance,
- In September 2023, the CDC updated its guidelines to emphasize the use of Class 5 chemical indicators and biological monitoring for steam sterilization in surgical settings, reinforcing the importance of advanced sterilization monitoring tools
- In 2022, a study published in the American Journal of Infection Control reported that facilities using real-time digital sterilization monitoring systems reduced their surgical site infection (SSI) rates by over 30%, underlining the clinical and operational benefits of these technologies
- The heightened focus on infection prevention, backed by evolving regulatory requirements, continues to drive robust growth in the global sterilization monitoring market
Opportunity
“Integration of IoT and Digital Technologies for Smart Sterilization Monitoring”
- The integration of Internet of Things (IoT) and digital technologies into sterilization monitoring systems presents a significant growth opportunity for the global market. These smart systems enable real-time data collection, wireless communication, and automated compliance reporting, enhancing the overall efficiency and safety of sterilization processes
- IoT-enabled sterilization monitoring devices can track cycle parameters, detect deviations instantly, and alert personnel in real-time, reducing the risk of human error and ensuring consistent sterilization outcomes across healthcare and laboratory environments
- In addition, Digital platforms also allow centralized data storage and easy access to sterilization records, which is essential for audits, traceability, and quality assurance, especially in large healthcare systems
For instance,
- In October 2024, according to a report by the Journal of Healthcare Engineering, IoT-integrated sterilization monitoring systems significantly improved sterilization cycle traceability and reduced reporting errors by over 40% in hospital sterilization departments, streamlining compliance with health regulations
- In June 2023, according to research published in the International Journal of Medical Informatics, hospitals using cloud-connected sterilization monitoring platforms experienced improved workflow efficiency and reduced equipment downtime due to predictive maintenance features powered by AI and IoT analytics
- The adoption of IoT and smart technologies in sterilization monitoring not only enhances operational efficiency but also supports a proactive approach to infection control, offering long-term benefits in terms of patient safety and regulatory compliance
Restraint/Challenge
“High Implementation and Maintenance Costs Limiting Adoption in Resource-Limited Settings”
- The high initial costs associated with advanced sterilization monitoring systems, along with ongoing maintenance and calibration expenses, pose a major challenge to widespread market adoption—particularly in low- and middle-income countries
- These systems often require significant investment in equipment, staff training, and infrastructure upgrades, which can be difficult for small healthcare facilities and rural hospitals with constrained budgets to afford
- The cost burden is further intensified when digital and automated monitoring systems are involved, as they necessitate integration with IT systems and compliance with cybersecurity standards
For instance,
- In August 2023, a report by the Global Health Observatory highlighted that many healthcare facilities in Sub-Saharan Africa and parts of Southeast Asia still rely on manual sterilization monitoring methods due to the prohibitive cost of modern systems, limiting their ability to meet international sterilization standards
- In March 2022, an article in the Journal of Infection Prevention noted that despite the clinical benefits of smart sterilization monitoring systems, high acquisition and maintenance costs remain the most cited barriers among healthcare administrators, particularly in smaller clinics and public hospitals
- Consequently, financial constraints continue to impede market penetration in cost-sensitive regions, potentially affecting infection control outcomes and contributing to regional disparities in healthcare quality and safety
Sterilization Monitoring Market Scope
The market is segmented on the basis of technology, product, mode of sterilization, process, and end user.
|
Segmentation |
Sub-Segmentation |
|
By Technology |
|
|
By Product |
|
|
By Mode of Sterilization |
|
|
By Process |
|
|
By End User |
|
Sterilization Monitoring Market Regional Analysis
“North America is the Dominant Region in the Sterilization Monitoring Market”
- North America holds the dominant share in the global sterilization monitoring market, driven by its advanced healthcare infrastructure, stringent regulatory requirements, and high adoption of sophisticated infection control technologies
- U.S. plays a crucial role, with widespread use of sterilization monitoring systems in hospitals, surgical centers, and dental clinics, supported by strong governmental regulations and well-established reimbursement policies
- As hospital-acquired infections (HAIs) remain a major concern, healthcare facilities across North America continue to invest in reliable sterilization technologies to meet regulatory standards and ensure patient safety
- In addition, increasing awareness around infection control and the need for compliance with healthcare guidelines, the market is further bolstered by continuous advancements in sterilization monitoring devices and systems
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- Asia-Pacific is expected to experience the highest growth rate in the sterilization monitoring market, fueled by rapid healthcare infrastructure development, rising awareness about infection prevention, and increasing healthcare expenditure
- Countries such as China, India, and Japan are emerging as key markets due to the growing number of healthcare facilities and the increasing focus on infection control across hospitals and laboratories
- Japan, with its advanced healthcare system, is a key player in the region, adopting the latest sterilization technologies to maintain high standards in infection prevention. The country's rigorous regulatory standards further drive the demand for advanced sterilization monitoring solutions
- China and India, with their large and expanding healthcare sectors, are witnessing a surge in sterilization monitoring adoption, supported by government initiatives to improve healthcare standards, enhance patient safety, and reduce infection rates. The growing private healthcare sector and expanding pharmaceutical industries in these countries are contributing to market growth
Sterilization Monitoring Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- 3M (U.S.)
- STERIS (Ireland)
- Getinge (Sweden)
- Medivators Inc. (U.S.)
- Cardinal Health (U.S.)
- Mesa Labs, Inc. (U.S.)
- Propper Manufacturing Co., Inc. (U.S.)
- ANTONIO MATACHANA, S. A. (Spain)
- H.W.Andersen Products Ltd. (U.K.)
- Terragene S.A. (Argentina)
- Tuttnauer (Netherlands)
- PMS Healthcare Technologies. (Turkey)
- Liofilchem S.r.l. (Italy)
- Andersen Sterilizers (U.S.)
- Sterigenics U.S., LLC (U.S.)
- ASP (U.S.)
- Belimed AG (Switzerland)
- Sotera Health Company (U.S.)
- Nordion (Canada)
- SGS Société Générale de Surveillance SA (Switzerland)
Latest Developments in Global Sterilization Monitoring Market
- In March 2024, the U.S. Environmental Protection Agency (EPA) introduced stricter regulations on ethylene oxide emissions from sterilization facilities. This decision aims to reduce exposure to the chemical, balancing health concerns with the need for effective sterilization in the medical device industry
- In October 2024, ASP Japan G.K. expanded its sterilization monitoring portfolio by launching new chemical and biological indicators. These products ensure the effectiveness of sterilization processes in healthcare facilities, meeting the growing demand for safety and efficiency
- In October 2024, Transparency Market Research reported that sterilization monitoring solutions are increasingly being integrated with digital platforms. This integration provides remote monitoring and real-time data management, improving the overall sterilization process in healthcare settings
- In October 2024, a new sterilization service offering incorporating telesterilization was unveiled, allowing real-time monitoring of sterilization processes from remote locations, enhancing operational efficiency in large healthcare networks
- In October 2024, industry stakeholders discussed the harmonization of sterilization standards at a global level. This regulatory effort helps streamline compliance for multinational companies, facilitating smoother market entry across regions
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.