Global Stills Disease Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
3.10 Billion
USD
4.58 Billion
2024
2032
USD
3.10 Billion
USD
4.58 Billion
2024
2032
| 2025 –2032 | |
| USD 3.10 Billion | |
| USD 4.58 Billion | |
|
|
|
|
Still's Disease Treatment Market Size
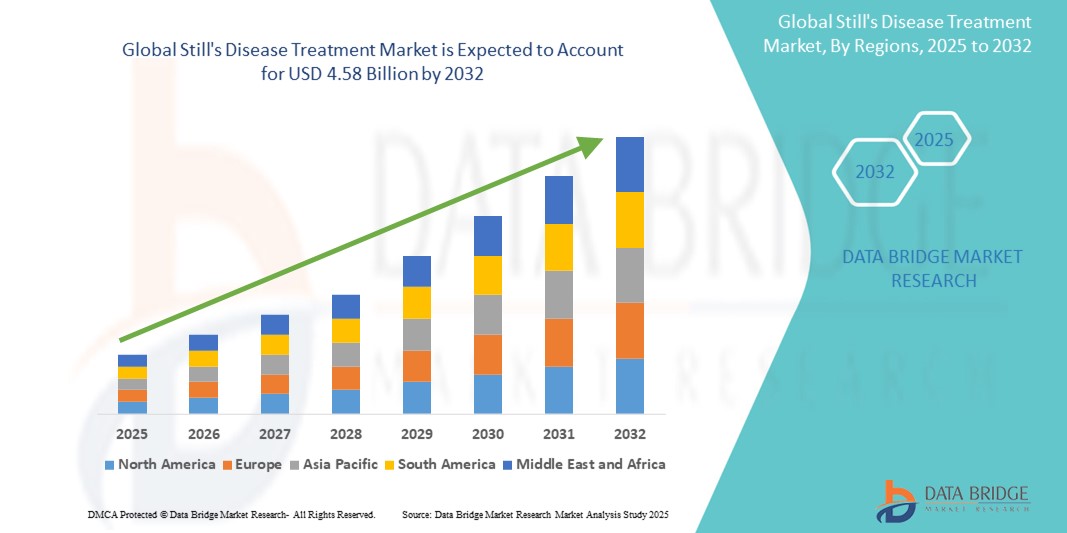
- The global still's disease treatment market size was valued at USD 3.10 billion in 2024 and is expected to reach USD 4.58 billion by 2032, at a CAGR of 5.05% during the forecast period
- This growth is driven by the increasing prevalence of autoimmune disorders, advancements in biologic therapies, and the expansion of diagnostic capabilities
Still's Disease Treatment Market Analysis
- Still's disease is a rare inflammatory condition that can present in both adult and systemic juvenile forms. Treatment primarily focuses on symptom management, inflammation control, and long-term immune modulation using a combination of NSAIDs, corticosteroids, conventional DMARDs, and biologics targeting IL-1 and IL-6 pathways
- The market is witnessing growth due to increased awareness, availability of targeted therapies, and growing access to specialty rheumatology care across emerging markets
- North America holds the largest share in the Still's Disease Treatment market with approximately 40.2%, supported by early diagnosis, clinical research, and access to innovative therapies
- Asia-Pacific is projected to register the highest CAGR due to expanding specialty clinics, greater disease recognition, and increased healthcare investments
- The Anakinra segment is expected to account for the largest market share of 43.1%, due to its widespread clinical use in both adult and pediatric Still’s Disease cases, rapid onset of action, and strong endorsement across treatment guidelines for IL-1 mediated inflammatory disorders
Report Scope and Still’s disease treatment Market Segmentation
|
Attributes |
Still’s Disease Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Still’s Disease Treatment Market Trends
" Growing Adoption of Personalized Biologic Therapies"
• The Still’s disease treatment market is witnessing a shift toward personalized biologic therapies, leveraging advanced biomarkers and genetic profiling to tailor IL-1 and IL-6 inhibitors like anakinra, canakinumab, and tocilizumab to individual patient profiles.
This approach optimizes dosing, minimizes side effects, and improves remission rates by targeting specific inflammatory pathways based on ferritin levels, cytokine profiles, and genetic predispositions. The trend aligns with the rising demand for precision medicine in autoimmune disorders, enhancing outcomes in both pediatric (SJIA) and adult (AOSD) populations. Integration of AI-driven diagnostic tools is further refining treatment plans, enabling real-time monitoring of inflammatory markers.
- For instance, in February 2025, a U.S.-based rheumatology consortium reported that biomarker-guided canakinumab dosing reduced flare rates by 70% in SJIA patients with elevated IL-1β, compared to standard protocols, in a multicenter study.
• The growing adoption of personalized biologic therapies, supported by biomarkers and AI diagnostics, is transforming Still’s disease treatment , enhancing efficacy and aligning with precision medicine trends to propel market growth.
Still’s disease treatment Market Dynamics
Driver
" Rising Burden of Autoimmune and Inflammatory Diseases"
- The increasing prevalence of autoimmune disorders, including Still’s Disease, is driven by genetic predisposition, environmental triggers, and improved diagnostic capabilities. Advanced biomarkers, such as elevated ferritin and IL-1/IL-6 levels, enable earlier diagnosis, expanding the treated population.
- This surge, particularly in developed regions, is boosting demand for targeted therapies like biologics (e.g., anakinra, tocilizumab). The growing recognition of Still’s Disease as a distinct entity, coupled with routine inflammatory marker testing, is increasing patient volumes in rheumatology clinics. Global awareness campaigns, supported by organizations like the International Still’s Disease Foundation, are encouraging early evaluation, further driving treatment demand.
- For Instance, In 2024, a European Rheumatology Association study reported a 10% rise in Still’s Disease diagnoses in the EU, attributed to enhanced biomarker screening in pediatric and adult populations.
- The rising prevalence and early diagnosis of Still’s Disease, fueled by advanced biomarkers and awareness, are significantly expanding the treatment market, driving demand for targeted therapies.
Opportunity
" Pediatric Focus and Regulatory Acceleration for Rare Indications"
- Orphan drug designations and pediatric research initiatives are spurring innovation in Still’s Disease therapies, particularly for Systemic Juvenile Idiopathic Arthritis (SJIA). Regulatory incentives, such as FDA and EMA fast-track pathways, accelerate approvals for biologics targeting rare indications. Biotech firms are prioritizing pediatric formulations to address unmet needs in young patients.
- Collaborative research, such as the CARRA registry, is generating real-world evidence to guide pediatric treatment protocols. These efforts are creating opportunities for novel IL-1/IL-6 inhibitors and combination therapies, enhancing market penetration in pediatric rheumatology.
- For Instance, In 2023, Novartis received FDA Breakthrough Therapy Designation for canakinumab in SJIA, following a Phase III trial showing a 65% reduction in systemic flares in children.
- Pediatric-focused research and regulatory incentives are catalyzing the development of Still’s Disease therapies, enhancing treatment options and driving market growth.
Restraint/Challenge
"Cost of Biologics and Delayed Diagnosis in LMICs"
- The high cost of biologics, such as anakinra and tocilizumab, often exceeding $50,000 annually, limits access in low- and middle-income countries (LMICs). Delayed diagnosis due to limited rheumatology expertise and biomarker testing further restricts timely treatment, worsening outcomes.
- Supply chain issues for temperature-sensitive biologics hinder consistent availability. Efforts like public-private partnerships aim to address affordability, but scalability remains limited, impacting market growth in underserved regions.
- For Instance, A 2023 WHO report noted that only 15% of LMIC hospitals had access to IL-6 biomarker assays, delaying Still’s Disease diagnosis by an average of 18 months.
- The high cost of biologics and delayed diagnoses in LMICs pose significant barriers to Still’s disease treatment , limiting market potential and requiring targeted interventions to improve access.
Still’s disease treatment Market Scope
The market is segmented on the basis of drugs, therapy type, mode of administration, end user, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
By Drugs |
|
|
By Therapy Type |
|
|
By Mode of Administration |
|
|
By End User |
|
|
By Distribution Channel
|
|
In 2025, the anakinra segment is projected to dominate the market with a largest share in therapy type segment
In 2025, the anakinra segment is projected to dominate the market with the 43.1%, due to its widespread clinical use in both adult and pediatric Still’s Disease cases, rapid onset of action, and strong endorsement across treatment guidelines for IL-1 mediated inflammatory disorders. Clinical guidelines from rheumatology associations and national rare disease programs consistently include Anakinra as a first-line or early-line treatment. In addition, increasing physician familiarity, ease of subcutaneous administration, and expanding insurance coverage for rare autoinflammatory diseases continue to boost its market uptake.
The hospital segment expected to account for the largest share during the forecast period in end user market
In 2025, the hospitals are expected to account for the largest market share of 53.5% due to their central role in delivering systemic treatments such as IL-inhibitor biologics, integrated infusion and monitoring infrastructure, and alignment with reimbursement programs for rare inflammatory disorders. Hospital-based infusion centers facilitate timely and supervised biologic therapy, while centralized diagnostic labs and electronic health systems ensure adherence to treatment protocols and insurance criteria. Their role in managing adverse effects and coordinating long-term care further reinforces their dominance in the end user segment.
Still’s Disease Treatment Market Regional Analysis
“North America Holds the Largest Share in the Still’s Disease Treatment Market”
- North America dominates the Still’s disease treatment market, accounting for an estimated 40.21% of the global market share in 2025. This leadership is driven by high clinical awareness, widespread availability of IL-1 and IL-6 targeted biologics, and comprehensive reimbursement coverage for rare autoimmune disorders
- The U.S. leads the region with an estimated 33.82% market share, supported by a robust regulatory environment including Orphan Drug and Fast Track designations from the FDA, and consistent funding for rare disease innovation through NIH programs. In addition, the U.S. biopharmaceutical ecosystem supports ongoing clinical trials for novel biologic therapies targeting systemic juvenile idiopathic arthritis (sJIA) and adult-onset Still's Disease (AOSD)
- Multi-center rare disease research frameworks, including the Autoinflammatory Diseases Clinical Research Network (ADCRN) and NIH-funded pediatric rheumatology consortia, have enhanced access to clinical trials and real-world data. These infrastructures accelerate market entry and adoption of novel biologics, such as canakinumab and anakinra
- National health strategies emphasizing early immunological profiling, digital rheumatology records, and patient advocacy efforts (e.g., Systemic JIA Foundation) further solidify North America’s dominance by facilitating early detection, patient-centric care, and greater treatment accessibility
“Asia-Pacific is Projected to Register the Highest CAGR in the Still’s Disease Treatment Market”
- The Asia-Pacific region is projected to grow at the highest compound annual growth rate (CAGR) in the Still’s disease treatment market and currently holds an estimated 21.5% market share in 2025
- This growth is propelled by increasing diagnosis of autoimmune conditions, enhanced access to biologic treatments, and rising public investment in orphan and inflammatory disease therapies
- China and India are at the forefront, leading initiatives such as expansion of pediatric autoimmune disease registries, newborn screening programs, and partnerships with international rheumatology alliances to improve treatment protocols for Still’s Disease
- Emerging biotech hubs in South Korea, Japan, and Singapore are bolstering regional innovation through monoclonal antibody development, clinical trials in immunology, and localized production of anti-inflammatory agents. These advancements are significantly shortening treatment access timelines
Still’s Disease Treatment Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Merck & Co., Inc. (U.S.)
- Bristol-Myers Squibb Company (U.S.)
- Pfizer Inc. (U.S.)
- GlaxoSmithKline plc (U.K.)
- AstraZeneca (U.K.)
- Eli Lilly and Company (U.S.)
- Amgen Inc. (U.S.)
- Novartis AG (Switzerland)
- Johnson & Johnson Services, Inc. (U.S.)
- AbbVie Inc. (U.S.)
- Sanofi S.A. (France)
- Takeda Pharmaceutical Co., Ltd. (Japan)
- Biocon Limited (India)
- Seagen Inc. (U.S.)
Latest Developments in Global Still’s disease treatment Market
- In January 2025, Novartis launched a new indication for canakinumab targeting refractory adult-onset Still's Disease, following robust Phase III clinical trial results. The data demonstrated a significant reduction in disease flares and improved long-term remission rates, prompting regulatory approvals in the U.S. and select European markets. This move strengthens Novartis’ biologics portfolio and expands therapeutic options for patients with difficult-to-treat systemic inflammation.
- In September 2024, Regeneron expanded its IL-1 research program to include systemic juvenile idiopathic arthritis (sJIA), aiming to target Still’s-like pathology at earlier stages of disease progression. The initiative focuses on developing next-generation IL-1 inhibitors with enhanced safety profiles and broader pediatric indications, supporting the company’s long-term vision in rare auto-inflammatory conditions.
- In July 2024, Sobi collaborated with the European Paediatric Rheumatology Society (PReS) to conduct a real-world observational study assessing the safety, tolerability, and clinical outcomes of anakinra in pediatric Still’s Disease patients. The study spans over 10 countries and involves over 400 children, marking one of the most comprehensive real-world evidence efforts for IL-1 inhibitors in this indication.
- In March 2024, Sanofi received orphan drug designation in Japan for its IL-6 inhibitor sarilumab for the treatment of systemic juvenile idiopathic arthritis with features of Still's Disease. This regulatory milestone supports Sanofi’s strategy to expand sarilumab into pediatric autoimmune conditions and strengthens its position in the Asian immunology market.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.