Global Supraventricular Tachycardia Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
325.93 Billion
USD
560.00 Billion
2025
2033
USD
325.93 Billion
USD
560.00 Billion
2025
2033
| 2026 –2033 | |
| USD 325.93 Billion | |
| USD 560.00 Billion | |
|
|
|
|
Supraventricular Tachycardia Treatment Market Size
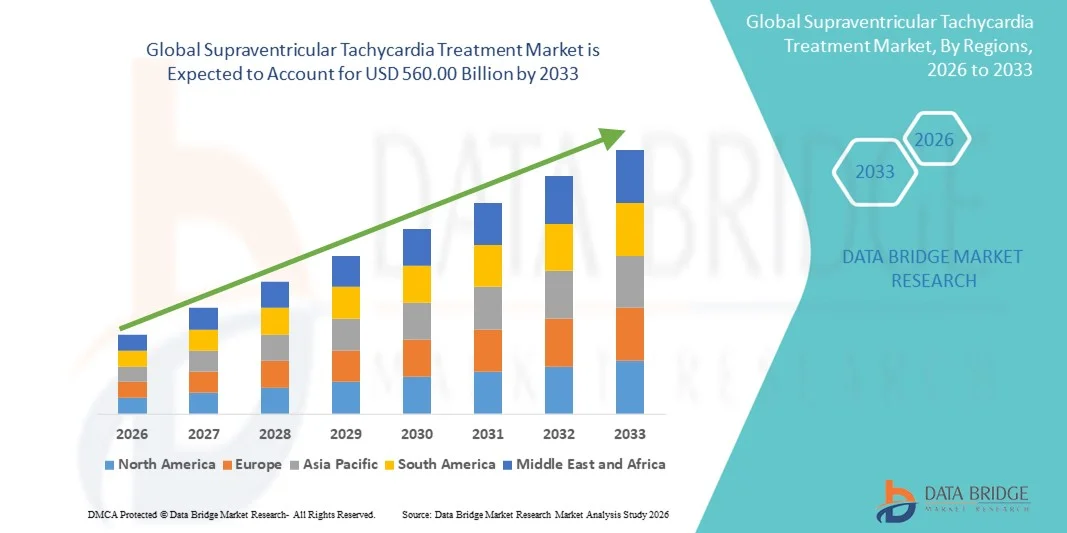
- The global supraventricular tachycardia treatment market size was valued at USD 325.93 billion in 2025 and is expected to reach USD 560.00 billion by 2033, at a CAGR of7.00% during the forecast period
- The market presents strong opportunity due to the rising prevalence of cardiovascular disorders and increasing awareness about early diagnosis and treatment of abnormal heart rhythms, leading to higher demand for advanced supraventricular tachycardia treatment solutions
- Furthermore, continuous advancements in minimally invasive procedures, catheter ablation technologies, and the development of novel antiarrhythmic drugs are creating significant growth opportunities for companies operating in the supraventricular tachycardia treatment market
Supraventricular Tachycardia Treatment Market Analysis
- Supraventricular Tachycardia Treatment, which includes medications such as beta-blockers, calcium channel blockers, anti-arrhythmic drugs, and catheter ablation procedures, has become increasingly critical in modern cardiology due to the rising prevalence of heart rhythm disorders and the growing need for rapid and effective heart rate control in both acute and chronic care settings
- The escalating demand for supraventricular tachycardia treatment is primarily fueled by increasing incidence of cardiovascular diseases, a growing geriatric population, improved access to emergency cardiac care, and rising awareness of early diagnosis and management of arrhythmias
- North America dominated the supraventricular tachycardia treatment market with the largest revenue share of 38.4% in 2025, supported by advanced healthcare infrastructure, high adoption of innovative cardiac technologies, strong reimbursement frameworks, and a high prevalence of cardiac disorders, particularly in the U.S., which continues to lead in the use of catheter ablation and advanced electrophysiology procedures
- Asia-Pacific is expected to be the fastest growing region in the supraventricular tachycardia treatment market during the forecast period due to rapidly improving healthcare infrastructure, rising healthcare expenditure, growing urbanization, and increasing awareness about cardiac health in emerging economies such as China and India
- The oral segment dominated the largest market revenue share of 58.3% in 2025, due to its non-invasive nature and convenience. Oral administration allows for long-term management of SVT without hospital visits. It is preferred for chronic patients and offers better compliance. Easy accessibility through pharmacies ensures wide distribution
Report Scope and Supraventricular Tachycardia Treatment Market Segmentation
|
Attributes |
Supraventricular Tachycardia Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
• Abbott (U.S.) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Supraventricular Tachycardia Treatment Market Trends
Advancements in Minimally Invasive and Targeted Therapeutic Approaches
- • A significant and accelerating trend in the global supraventricular tachycardia (SVT) treatment market is the growing shift toward minimally invasive procedures and more targeted pharmacological therapies aimed at improving patient outcomes and reducing complication risks. This evolution is driven by advancements in cardiac electrophysiology, improved diagnostic accuracy, and increased adoption of catheter-based interventions.
- For instance, in March 2023, Medtronic’s Affera Mapping and Ablation System (with its Sphere-9 catheter) received CE Mark approval for the treatment of atrial arrhythmias, combining high-density mapping and dual-energy ablation in a single device — a development that underscores the move toward more efficient and integrated ablation platforms
- Simultaneously, the development of newer antiarrhythmic drugs and beta-blockers with improved safety profiles is supporting more effective long-term management of SVT. These drugs are designed to control heart rhythm with fewer side effects, thereby improving patient compliance and reducing the frequency of emergency interventions related to SVT episodes.
- The increasing integration of advanced diagnostic tools such as 3D electroanatomical mapping, wearable heart rhythm monitors, and portable ECG devices is also contributing to early detection and continuous monitoring of SVT. These technologies enable physicians to tailor treatment strategies based on real-time patient data and improve overall disease management
- The growing awareness of heart rhythm disorders, combined with enhanced access to specialized cardiology services, is encouraging earlier interventions and proactive treatment planning. This trend is fundamentally reshaping the management of SVT by prioritizing long-term rhythm control, reduced hospitalization, and improved quality of life for patients worldwide
- As a result, pharmaceutical companies and medical device manufacturers are increasingly focusing on innovation in antiarrhythmic medications, ablation technologies, and monitoring devices, creating a more comprehensive and efficient treatment ecosystem for Supraventricular Tachycardia
Supraventricular Tachycardia Treatment Market Dynamics
Driver
Rising Prevalence of Cardiac Arrhythmias and Improving Access to Cardiac Care
- The increasing global prevalence of cardiac arrhythmias, including Supraventricular Tachycardia, is a primary driver of market growth. Factors such as aging populations, rising rates of cardiovascular disease, high stress levels, obesity, and sedentary lifestyles are contributing to a growing number of SVT cases across both developed and emerging economies
- For instance, in December 2023, Medtronic’s PulseSelect Pulsed-Field Ablation (PFA) System received FDA approval for treatment of atrial fibrillation, making it one of the first PFA systems in the U.S. Such newer energy-delivery systems have the potential to be adapted for SVT ablation in the future, increasing the attractiveness of advanced electrophysiology treatment centers
- Increased awareness among patients regarding the symptoms of abnormal heart rhythms, such as rapid heartbeat, dizziness, chest discomfort, and shortness of breath, is encouraging earlier medical consultations. This, in turn, is resulting in a higher rate of diagnosis and intervention
- Furthermore, the availability of portable heart monitoring technologies and improved screening initiatives is helping to identify SVT at earlier stages, even in asymptomatic individuals. This proactive approach is supporting an overall increase in treatment volume in both hospital and outpatient environments
- The growing emphasis on preventive cardiology and routine cardiovascular check-ups is also contributing to market expansion, as more individuals are being diagnosed with arrhythmias before the condition progresses into severe complications
Restraint/Challenge
High Cost of Advanced Procedures and Limited Access in Rural Areas
- Despite technological advancements, the high cost of advanced SVT treatments, particularly catheter ablation procedures and long-term medication regimens, remains a major barrier to market penetration. These treatments often require specialized equipment, skilled professionals, and hospital infrastructure, which can make them financially inaccessible for a large segment of the population
- For instance, Medtronic’s new Affera system with the Sphere-9 catheter (which streamlines mapping and ablation) has a complex, high-density mapping platform that may increase procedural costs and require investment in specialized EP labs — potentially limiting adoption in hospitals with constrained budgets
- In many developing and underdeveloped regions, limited availability of electrophysiology laboratories and trained cardiologists further restricts access to effective treatment. Patients in rural and semi-urban areas often must travel long distances to receive specialized cardiac care, leading to delays in diagnosis and reduced treatment rates
- In addition, some patients experience hesitancy toward invasive procedures due to fear, lack of awareness, or cultural perceptions, which can result in underutilization of highly effective treatment options such as catheter ablation
- Variability in reimbursement policies and limited insurance coverage for advanced cardiac procedures also present challenges, particularly in low- and middle-income countries. Out-of-pocket expenses for treatment, diagnostic tests, and follow-up care can discourage patients from pursuing complete and consistent treatment
- Overcoming these challenges will require increased government funding, improved healthcare infrastructure in underserved areas, broader insurance coverage, and enhanced educational initiatives aimed at promoting awareness and early intervention for supraventricular tachycardia
Supraventricular Tachycardia Treatment Market Scope
The market is segmented on the basis of drug type, treatment, diagnosis, dosage, route of administration, end-users, and distribution channel.
- By Drug Type
On the basis of drug type, the Supraventricular Tachycardia Treatment market is segmented into antiarrhythmic drugs and others. The antiarrhythmic drugs segment dominated the largest market revenue share of 56.8% in 2025, driven by its position as the frontline treatment option for the management of supraventricular tachycardia. These drugs are widely prescribed to control abnormal electrical signals in the heart and stabilize irregular rhythms. They are commonly used in both acute emergency situations and for long-term rhythm management. The availability of multiple drug classes such as beta-blockers, calcium channel blockers, and sodium channel blockers has increased treatment flexibility. Strong clinical evidence supporting their efficacy, combined with physician familiarity, continues to boost their adoption. Their cost-effectiveness compared to invasive procedures further strengthens market penetration. Hospitals and clinics largely depend on these drugs for immediate symptom relief. Growing awareness about early diagnosis and treatment of arrhythmias also contributes to increased prescription rates. In addition, the wide availability of generic formulations makes these drugs more accessible in developing regions. All these factors collectively reinforce the dominance of the antiarrhythmic drugs segment in the global market.
The others segment is expected to witness the fastest CAGR of 10.6% from 2026 to 2033, driven by the increasing development of advanced supportive therapies and emerging drug classes. Continuous research focuses on reducing side effects and improving long-term patient outcomes. Growing investments in novel pharmacological approaches for patients unresponsive to conventional treatment are supporting this segment’s expansion. The rising trend toward personalized medicine is also driving demand for alternative therapies. Increasing collaborations between pharmaceutical companies and research institutes are accelerating innovation. Improved regulatory approvals for newer therapies are enhancing acceptance. As awareness about treatment-resistant SVT cases grows, physicians are turning to these options more frequently. This trend is expected to significantly boost the market share of this segment in the forecast period.
- By Treatment
On the basis of treatment, the Supraventricular Tachycardia Treatment market is segmented into carotid sinus massage, medication, cardioversion, vagal maneuvers, catheter ablation, pacemaker, and others. The medication segment dominated the largest market revenue share of 48.5% in 2025, due to its widespread use as the initial line of treatment in both outpatient and inpatient settings. Medications provide quick relief from rapid heart rates without the need for surgical intervention. They are effective in stabilizing patients and preventing recurrence of symptoms. The availability of a wide range of reliable drugs makes this treatment approach highly accessible across all healthcare facilities. Medication-based therapy is often preferred for elderly patients and those not eligible for invasive procedures. High physician confidence, lower treatment cost, and ease of administration drive the adoption of this segment. Hospitals rely heavily on drug therapy in emergency departments for immediate heart rate control. Long-term management through medications also ensures consistent demand. Increasing prevalence of SVT due to lifestyle-related factors has further accelerated growth. Combined with improving drug formulations, the medication segment continues to dominate the market strongly.
The catheter ablation segment is anticipated to witness the fastest CAGR of 12.3% from 2026 to 2033 due to its high success rate in permanently eliminating abnormal electrical pathways. This procedure is increasingly preferred by younger patients as it offers a long-term solution with minimal recurrence. Technological advancements in catheter design and 3D heart mapping systems are improving treatment precision. Shorter recovery times and growing acceptance of minimally invasive techniques are encouraging adoption. Rising awareness among patients regarding permanent treatment options is also boosting demand. The expansion of advanced cardiac hospitals globally is improving access to this procedure. Increased availability of trained specialists further supports growth. As a result, catheter ablation is emerging as a rapidly expanding segment within the overall market.
- By Diagnosis
On the basis of diagnosis, the Supraventricular Tachycardia Treatment market is segmented into electrocardiogram, echocardiogram, implantable loop recorder, holter monitor, and others. The electrocardiogram (ECG) segment dominated the largest market revenue share of 46.2% in 2025, as it is the most commonly used and widely available diagnostic tool. ECG provides quick, accurate, and real-time readings of the heart’s electrical activity. It is routinely used in emergency departments and outpatient clinics to detect abnormal heart rhythms. The non-invasive nature and low cost of ECG testing make it highly accessible. Portable and wearable ECG devices have further expanded its usage beyond clinical environments. Growing awareness of cardiac health is increasing regular screenings using this method. Integration with digital systems and AI-enabled analysis is also improving diagnostic accuracy. Hospitals rely on ECG as the primary tool for SVT confirmation. Its ability to deliver immediate results makes it indispensable in time-sensitive cases. These factors contribute to its continued dominance.
The implantable loop recorder segment is projected to grow at the fastest CAGR of 11.4% from 2026 to 2033, supported by the rising need for long-term heart rhythm monitoring. This device is especially useful for patients experiencing intermittent symptoms. Continuous monitoring over months or years helps in accurate diagnosis of complex cases. Technological improvements have made these devices smaller and more comfortable for patients. Increased recommendations from cardiologists are accelerating usage. Growing awareness of undiagnosed arrhythmias is also boosting demand. As remote patient monitoring expands, this segment will see substantial growth in the coming years.
- By Dosage
On the basis of dosage, the Supraventricular Tachycardia Treatment market is segmented into tablet, injection, and others. The tablet segment dominated the largest market revenue share of 52.6% in 2025, due to its convenience and ease of administration. Oral tablets are widely used for long-term management of heart rhythm disorders. They offer high patient compliance, particularly among elderly and chronic patients. Tablets are easy to distribute through retail and hospital pharmacies. Lower cost compared to injectable formulations further increases their popularity. Patients can easily continue therapy at home, supporting consistent demand. The availability of extended-release formulations has also improved treatment efficiency. Growing preference for non-invasive treatment options supports this segment’s dominance. Increased outpatient treatments worldwide further boost tablet consumption. Physicians commonly prescribe oral medications as maintenance therapy after initial stabilization. All these factors sustain its strong hold in the market.
The injection segment is expected to witness the fastest CAGR of 9.8% from 2026 to 2033, primarily due to its rapid effectiveness in emergency situations. Injections are commonly used in hospitals when an immediate response is required. The growing number of emergency cardiac admissions is driving usage. Advanced injectable drugs are being introduced with better safety profiles. Increasing investment in hospital infrastructure is expanding accessibility. The need for quick heart rate stabilization supports higher demand for injectable forms. As critical care units expand globally, this segment is set to experience significant growth.
- By Route of Administration
On the basis of route of administration, the Supraventricular Tachycardia Treatment market is segmented into oral, intravenous, and others. The oral segment dominated the largest market revenue share of 58.3% in 2025, due to its non-invasive nature and convenience. Oral administration allows for long-term management of SVT without hospital visits. It is preferred for chronic patients and offers better compliance. Easy accessibility through pharmacies ensures wide distribution. Lower cost and minimal discomfort make it highly acceptable. Growing awareness of early and continuous treatment further boosts its adoption. Oral medications also support outpatient care models. Patients find it easier to maintain daily routines with oral therapy. The availability of multiple drug options strengthens this segment’s position. These factors keep oral administration at the forefront of the market.
The intravenous segment is anticipated to witness the fastest CAGR of 10.1% from 2026 to 2033, attributed to its use in critical and emergency care. IV administration provides immediate drug action, essential in severe cases. Growing emergency response capabilities enhance its usage. Technological improvements in IV drug delivery systems improve safety and efficiency. Increased hospital admissions related to heart disorders further support growth. With the rising prevalence of acute cardiac episodes, the demand for IV administration is set to rise significantly.
- By End-Users
On the basis of end-users, the Supraventricular Tachycardia Treatment market is segmented into clinic, hospital, and others. The hospital segment dominated the largest market revenue share of 49.7% in 2025, driven by the availability of advanced diagnostics and specialized care. Hospitals serve as the primary centers for SVT treatment, especially in emergency cases. Presence of cardiologists and advanced equipment enhances treatment accuracy. Hospitals also perform advanced procedures such as catheter ablation and cardioversion. The increasing number of multi-specialty hospitals boosts this segment. Government investments in healthcare infrastructure further strengthen this position. High patient inflow with cardiac conditions maintains constant demand. Availability of intensive care units ensures immediate intervention. These factors contribute to strong hospital dominance worldwide.
The clinic segment is expected to witness the fastest CAGR of 9.5% from 2026 to 2033, due to growing outpatient visits and early diagnosis trends. Clinics provide easy access and shorter waiting times. Rising awareness encourages people to seek early consultation. Expansion of specialized cardiac clinics supports this growth. The adoption of portable diagnostic devices in clinics also increases demand. Improved healthcare access in suburban and rural areas further drives this segment. Increasing government initiatives to strengthen primary healthcare infrastructure is also contributing to higher patient footfall in clinics. In addition, partnerships between clinics and diagnostic centers are enhancing service availability and accelerating segment growth.
- By Distribution Channel
On the basis of distribution channel, the Supraventricular Tachycardia Treatment market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The hospital pharmacy segment dominated the largest market revenue share of 44.9% in 2025, supported by the immediate availability of medicines in emergency and inpatient settings. Hospital pharmacies dispense critical drugs used during surgical procedures and acute treatment. Integration with hospital care systems increases efficiency. Availability of specialized medications enhances its importance. Continuous patient inflow maintains steady demand. Hospitals also store high-value injectables and critical drugs. Strong regulatory control improves drug safety and trust. These factors contribute significantly to its dominant position in the market.
The online pharmacy segment is expected to witness the fastest CAGR of 13.2% from 2026 to 2033, due to the growth in digital healthcare. Online platforms offer convenience, quick access, and home delivery services. Rising smartphone usage and internet penetration support this trend. Increased preference for remote purchasing boosts demand. Competitive pricing and subscription models further accelerate growth. As e-commerce expands in healthcare, this channel is set to rise rapidly. The availability of detailed product information and consultation chat support is increasing consumer confidence. Improved logistics networks and faster delivery times are also strengthening adoption. In addition, the expansion of telemedicine services is driving higher prescription fulfillment through online platforms.
Supraventricular Tachycardia Treatment Market Regional Analysis
- North America dominated the supraventricular tachycardia treatment market with the largest revenue share of 38.4% in 2025
- Supported by advanced healthcare infrastructure, high adoption of innovative cardiac technologies, strong reimbursement frameworks, and a high prevalence of cardiac disorders, particularly in the U.S., which continues to lead in the use of catheter ablation and advanced electrophysiology procedures
- The region also benefits from strong investments in cardiac research, a well-established network of specialty clinics and hospitals, and high awareness of heart rhythm disorders among both patients and healthcare professionals
U.S. Supraventricular Tachycardia Treatment Market Insight
The U.S. supraventricular tachycardia treatment market captured the largest revenue share within North America in 2025, driven by the high prevalence of arrhythmia-related conditions, increasing use of minimally invasive treatment options such as catheter ablation, and rapid integration of advanced cardiac diagnostic and monitoring technologies. The presence of key pharmaceutical and medical device companies, along with favorable reimbursement policies and early adoption of innovative therapies, continues to support strong market expansion.
Europe Supraventricular Tachycardia Treatment Market Insight
The Europe supraventricular tachycardia treatment market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by rising geriatric population, increasing cases of cardiovascular disorders, and growing focus on preventive healthcare. Technological advancements in diagnosis and treatment, coupled with improving access to specialized cardiac care across countries such as Germany, France, and the U.K., are further supporting market growth in the region.
U.K. Supraventricular Tachycardia Treatment Market Insight
The U.K. supraventricular tachycardia treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by expanding cardiac care programs within the NHS, increased awareness of arrhythmia management, and rising demand for effective long-term treatment options. Growing investments in digital health and remote cardiac monitoring systems are also contributing to improved diagnosis and management of SVT in the country.
Germany Supraventricular Tachycardia Treatment Market Insight
The Germany supraventricular tachycardia treatment market is expected to expand at a considerable CAGR during the forecast period, supported by its strong medical technology base, high healthcare expenditure, and continuous innovation in cardiac imaging and electrophysiology. The country’s focus on precision medicine and early detection of heart rhythm abnormalities is further driving demand for advanced SVT treatment solutions.
Asia-Pacific Supraventricular Tachycardia Treatment Market Insight
Asia-Pacific supraventricular tachycardia treatment market is expected to be the fastest growing region in the supraventricular tachycardia treatment market during the forecast period, due to rapidly improving healthcare infrastructure, rising healthcare expenditure, growing urbanization, and increasing awareness about cardiac health in emerging economies such as China and India. The expanding middle-class population, improving access to modern healthcare facilities, and increasing government initiatives aimed at strengthening cardiovascular care services are significantly boosting the adoption of advanced SVT diagnostic and treatment options across the region
Japan Supraventricular Tachycardia Treatment Market Insight
The Japan supraventricular tachycardia treatment market is gaining momentum due to the country’s advanced healthcare system, aging population, and strong focus on early disease detection and management. The increasing use of cutting-edge medical devices for cardiac rhythm monitoring, along with widespread availability of specialized cardiac treatment centers, is accelerating market growth.
China Supraventricular Tachycardia Treatment Market Insight
The China supraventricular tachycardia treatment market accounted for the largest revenue share in Asia-Pacific in 2025, driven by rapid urbanization, rising prevalence of cardiovascular diseases, expanding healthcare coverage, and increasing adoption of advanced medical technologies. The growing number of hospitals, improvement in cardiac care services, and rising awareness of heart health are key factors propelling strong market demand in the country.
Supraventricular Tachycardia Treatment Market Share
The Supraventricular Tachycardia Treatment industry is primarily led by well-established companies, including:
• Abbott (U.S.)
• Medtronic (Ireland)
• Boston Scientific Corporation (U.S.)
• Johnson & Johnson (U.S.)
• Pfizer Inc. (U.S.)
• Novartis AG (Switzerland)
• AstraZeneca (U.K.)
• Sanofi (France)
• GlaxoSmithKline plc (U.K.)
• Bayer AG (Germany)
• Bristol Myers Squibb (U.S.)
• Roche Holding AG (Switzerland)
• Merck & Co., Inc. (U.S.)
• Siemens Healthineers (Germany)
• GE HealthCare (U.S.)
• Koninklijke Philips N.V. (Netherlands)
• Teva Pharmaceutical Industries Ltd. (Israel)
• Hikma Pharmaceuticals PLC (U.K.)
• Cipla Ltd. (India)
• Sun Pharmaceutical Industries Ltd. (India)
Latest Developments in Global Supraventricular Tachycardia Treatment Market
- In December 2023, Medtronic received FDA approval for its PulseSelect Pulsed Field Ablation (PFA) System, becoming the first company to gain U.S. approval for a pulsed field ablation technology in cardiac arrhythmia treatment. This advanced system uses non-thermal electrical energy to selectively target abnormal heart tissue while minimizing damage to surrounding structures such as the esophagus and nerves. Although initially approved for atrial fibrillation, the same PFA technology platform is being increasingly evaluated for supraventricular tachycardia (SVT), and its introduction represents a major technological leap in catheter ablation procedures
- In January 2024, Boston Scientific secured FDA approval for its FARAPULSE Pulsed Field Ablation System, based on strong clinical trial data demonstrating safety, precision, and shorter procedure times compared to traditional thermal ablation technologies. FARAPULSE delivers ultra-fast energy in a highly controlled manner, enabling physicians to perform rapid and effective ablation. This innovation is extremely relevant to the treatment of SVT, as electrophysiology labs can now apply this same next-generation platform to a broader range of arrhythmias beyond atrial fibrillation
- In November 2024, Johnson & Johnson MedTech (Biosense Webster) obtained FDA approval for its VARIPULSE PFA Platform, which integrates with its CARTO 3 mapping system. This provides physicians with improved real-time visualization and precision guidance when targeting arrhythmogenic tissue. The integration of advanced 3D mapping and next-generation ablation energy significantly enhances treatment accuracy in complex supraventricular tachycardia cases, strengthening J&J’s position in the global electrophysiology and SVT treatment market
- In September 2024, Boston Scientific received regulatory approval in Japan for the FARAPULSE System, marking a major geographic expansion of this innovative technology into Asia. This approval enabled hospitals and cardiac centers in Japan to begin adopting pulsed field ablation for arrhythmia treatment, including supraventricular tachycardia, reflecting the growing global acceptance of next-generation catheter ablation systems
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.