Global Syphilis Immunoassay Diagnostics Market
Market Size in USD Million
CAGR :
% 
 USD
579.64 Million
USD
1.03 Million
2024
2032
USD
579.64 Million
USD
1.03 Million
2024
2032
| 2025 –2032 | |
| USD 579.64 Million | |
| USD 1.03 Million | |
|
|
|
|
Syphilis Immunoassay Diagnostics Market Size
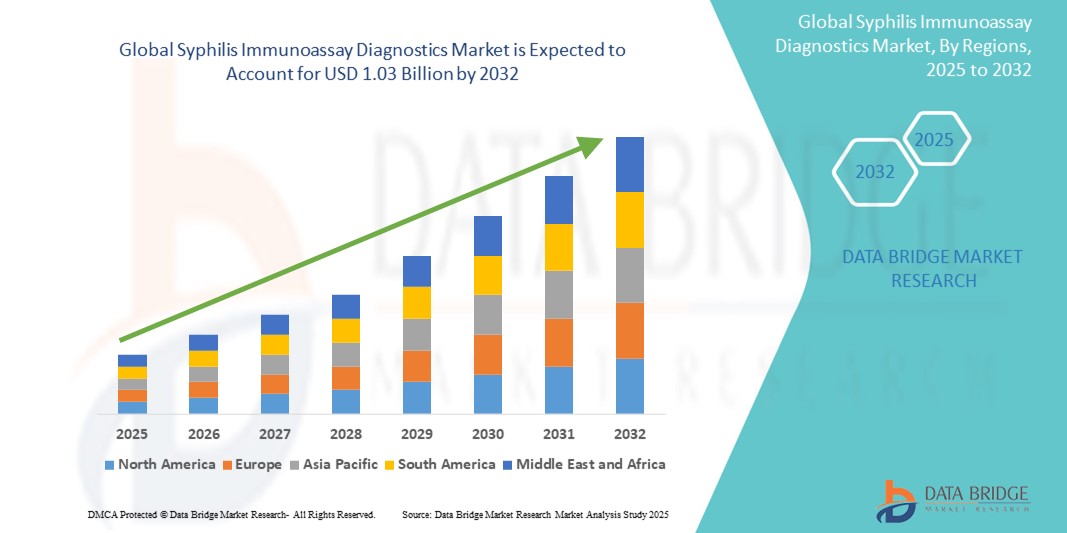
- The global syphilis immunoassay diagnostics market size was valued at USD 579.64 million in 2024 and is expected to reach USD 1.03 billion by 2032, at a CAGR of 7.60% during the forecast period
- This growth is driven by factors such as the increasing prevalence of syphilis and other sexually transmitted infections (stis), and advancements in diagnostic technologies
Syphilis Immunoassay Diagnostics Market Analysis
- Syphilis immunoassay diagnostics are crucial tools used for the detection and diagnosis of syphilis, an increasingly prevalent sexually transmitted infection (STI). These tests provide accurate results and are essential for early diagnosis and treatment to prevent further complications
- The demand for syphilis immunoassay diagnostics is significantly driven by the rising global prevalence of syphilis and other sexually transmitted infections (STIs), as well as advancements in diagnostic technologies. In addition, the growing focus on early detection, public health initiatives, and improving access to healthcare contribute to the increasing adoption of these diagnostic tools
- North America is expected to dominate the syphilis immunoassay diagnostics market with a market share of 42.6% globally. The region’s dominance is attributed to its advanced healthcare infrastructure, high adoption of innovative diagnostic technologies, and strong market presence of key players
- Asia-Pacific is expected to be the fastest growing region in the syphilis immunoassay diagnostics market with market share of 17.3% in the syphilis immunoassay diagnostics market, driven by factors such as expanding healthcare infrastructure, increasing awareness about sexually transmitted infections, and government-led public health initiatives
- The chemiluminescence immunoassays (CLIA) technology segment is expected to dominate the syphilis immunoassay diagnostics market with the largest share of 45.3% due to its high sensitivity, rapid detection capabilities, and suitability for automation in clinical settings, driving its increased adoption for syphilis testing in diagnostic labs
Report Scope and Syphilis Immunoassay Diagnostics Market Segmentation
|
Attributes |
Syphilis Immunoassay Diagnostics Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Syphilis Immunoassay Diagnostics Market Trends
“Advancements in Syphilis Immunoassay Diagnostics & Point-of-Care Testing”
- One prominent trend in the evolution of syphilis immunoassay diagnostics is the integration of rapid, point-of-care (POC) testing solutions that allow for quicker, more accurate detection in various settings
- These innovations enhance diagnostic accuracy by enabling faster results, which is crucial for timely treatment and prevention of transmission
- For instance, modern syphilis immunoassays now offer multiplex testing capabilities, allowing for simultaneous detection of syphilis and other STIs, improving efficiency and reducing the time patients wait for results
- These advancements are revolutionizing syphilis diagnosis, improving patient outcomes through early detection, and driving the demand for next-generation diagnostic tools that offer faster and more convenient testing options
Syphilis Immunoassay Diagnostics Market Dynamics
Driver
“Growing Need Due to Rising Prevalence of Sexually Transmitted Infections (STIs)”
- The increasing prevalence of syphilis, especially in high-risk populations, is significantly driving the demand for syphilis immunoassay diagnostics
- Syphilis rates have been on the rise globally, and with the growing awareness of the disease’s complications—such as neurological and cardiovascular damage—the need for timely and accurate diagnostic tools has become critical
- As more individuals seek testing, the demand for immunoassay diagnostics increases, providing healthcare professionals with reliable tools for early detection and treatment
For instance,
- In 2023, the World Health Organization (WHO) reported a 10% rise in syphilis cases globally, with a particular increase in syphilis among men who have sex with men (MSM), further driving the need for effective diagnostic solutions
- As a result of the rising prevalence of syphilis and other STIs, there is a significant increase in the demand for syphilis immunoassay diagnostics, helping to reduce transmission and improve public health outcomes
Opportunity
“Advancements in Point-of-Care Testing and Rapid Diagnostics”
- Recent innovations in rapid syphilis immunoassay tests, including point-of-care (POC) solutions, enable quick and accurate diagnosis outside traditional laboratory settings
- These advancements allow for faster diagnosis and treatment, particularly in remote and underserved areas, where access to laboratories may be limited
For instance,
- In December 2024, a new rapid syphilis immunoassay diagnostic device was launched in South Africa, designed to deliver results in under 20 minutes, significantly improving the accessibility and effectiveness of syphilis screening
- The integration of AI and multiplex testing in syphilis immunoassays also presents an opportunity to expand testing for other sexually transmitted infections (STIs), providing more comprehensive diagnostics and improving patient outcomes
Restraint/Challenge
“High Cost of Diagnostic Devices Limiting Market Accessibility”
- One significant challenge for the growth of the syphilis immunoassay diagnostics market is the high cost of diagnostic devices, which can limit their accessibility, particularly in low- and middle-income countries
- The cost of advanced immunoassay diagnostic kits may be prohibitive for some healthcare facilities and communities, leading to delayed diagnoses and treatment
For instance,
- In 2024, according to a report by the Global Fund, the high price of syphilis diagnostic tests in sub-Saharan Africa has hindered widespread screening programs, resulting in higher rates of undiagnosed cases
- This financial barrier affects the adoption of newer diagnostic technologies, reducing the overall penetration of syphilis immunoassays in key regions, and hampering efforts to control the spread of syphilis globally
Syphilis Immunoassay Diagnostics Market Scope
The market is segmented on the basis of technology, application and end user.
|
Segmentation |
Sub-Segmentation |
|
By Technology |
|
|
By Application |
|
|
By End User |
|
In 2025, the chemiluminescence immunoassays is projected to dominate the market with a largest share in technology segment
The chemiluminescence immunoassays technology segment is expected to dominate the Syphilis Immunoassay Diagnostics market with the largest share of 45.3% due to its high sensitivity, rapid detection capabilities, and suitability for automation in clinical settings, driving its increased adoption for syphilis testing in diagnostic labs.
The screening is expected to account for the largest share during the forecast period in application market
In 2025, the screening segment is expected to account for the largest share in the syphilis immunoassay diagnostics market, with 38.2% market share, as these tests are crucial for early detection and management of syphilis, particularly in high-risk populations and regions with limited access to healthcare facilities.
Syphilis Immunoassay Diagnostics Market Regional Analysis
“North America Holds the Largest Share in the Syphilis Immunoassay Diagnostics Market”
- North America dominates the syphilis immunoassay diagnostics market, driven by advanced healthcare infrastructure, high adoption of cutting-edge medical technologies, and strong presence of key market players
- North America is expected to dominate the syphilis immunoassay diagnostics market with a market share of 42.6% globally. The region’s dominance is attributed to its advanced healthcare infrastructure, high adoption of innovative diagnostic technologies, and strong market presence of key players
- U.S. is projected to hold the largest share in the North American market, with an estimated share of 68.5%. This dominance is driven by the country's robust healthcare system, high prevalence of syphilis, and extensive public health initiatives aimed at controlling and diagnosing sexually transmitted infections (STIs)
“Asia-Pacific is Projected to Register the Highest CAGR in the Syphilis Immunoassay Diagnostics Market”
- Asia-Pacific is expected to witness the highest growth rate with 17.3% in the syphilis immunoassay diagnostics market, This growth is driven by several key factors, including increasing awareness about sexually transmitted infections, rising government and NGO-led screening initiatives, and improving healthcare infrastructure across emerging economies such as India and China
- The growing population, expanding access to diagnostic facilities, and a rising burden of untreated or undiagnosed syphilis cases contribute to the region’s rapid market expansion.
- Technological advancements and the introduction of affordable, rapid testing kits are also enhancing the adoption of syphilis screening tools in both urban and rural settings. As public health efforts intensify, the Asia-Pacific region is expected to outpace other regions in market growth for syphilis immunoassay diagnostics.
- India is projected to register the highest CAGR with 5.7% market share in the Syphilis Immunoassay Diagnostics market, driven by factors such as expanding healthcare infrastructure, increasing awareness about sexually transmitted infections, and government-led public health initiatives
Syphilis Immunoassay Diagnostics Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Abbott (U.S.)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Siemens (Germany)
- BD (U.S.)
- Bio-Rad Laboratories, Inc. (U.S.)
- Ortho Clinical Diagnostics (U.S.)
- Hologic Inc. (U.S.)
- Beckman Coulter Inc. (U.S.)
- Thermo Fisher Scientific (U.S.)
- Cepheid (U.S.)
- MedMira Inc. (Canada)
- Kinghawk Pharmaceutical (China)
- Jalor Biotech (South Korea)
- Chembio Diagnostics (U.S.)
- Alere Inc. (U.S.)
- SDBioScience (China)
- Maccura Biotechnology (China)
- Trinity Biotech (Ireland)
- Nantong Egens Biotechnology (China)
- Wondfo Biotech (China)
Latest Developments in Global Syphilis Immunoassay Diagnostics Market
- In November 2024, NOWDiagnostics launched nationwide retail distribution of its First To Know Syphilis Test. The self-test became available in thousands of U.S. pharmacies, retail stores, and online platforms such as Amazon. At an estimated cost of USD 30, the test offers an affordable and accessible at-home option amid rising syphilis cases
- In October 2024, Chembio Diagnostics promoted its point-of-care syphilis testing solutions at the 2024 SCASM (La Jolla, CA) and the National Conference on Correctional Health Care (Las Vegas, NV). The company showcased its DPP® rapid tests, including the HIV-Syphilis combo, highlighting onsite screening benefits for acute care and correctional health environments
- In October 2024, NOWDiagnostics and Labcorp announced a distribution partnership. Labcorp became the exclusive U.S. distributor of the First To Know Syphilis Test across clinical and professional settings. The 15-minute test is expected to reach healthcare providers by the end of 2024 and be made available to patients via Labcorp OnDemand in 2025
- In September 2024, Beckman Coulter announced FDA 510(k) clearance for its Access Syphilis assay. The two-step, chemiluminescent microparticle immunoassay detects total antibodies to T. pallidum (IgG/IgM) in serum or plasma and runs on Beckman’s Access analyzers. The ready-to-use liquid format supports both screening and confirmatory protocols
- In August 2024, NOWDiagnostics received FDA marketing authorization for its First To Know Syphilis Test, the first at-home, over-the-counter syphilis antibody test in the U.S. The test uses one drop of blood from a finger-prick to detect total T. pallidum IgG/IgM, delivering results in about 15 minutes. Clinical studies showed ~99.5% agreement on negatives and ~93.4% on positives compared to laboratory-based assays
- In August 2024, QuidelOrtho Corporation (formerly Ortho Clinical Diagnostics) obtained FDA 510(k) clearance for its VITROS® Syphilis assay. This automated chemiluminescent immunoassay, compatible with VITROS 3600/5600/XT 7600 analyzers, detects total antibodies to T. pallidum. It supports the CDC-recommended reverse-testing algorithm and enhances laboratory efficiency
- In February 2023, Chembio Diagnostics (Nasdaq: CEMI) announced the FDA CLIA waiver of its DPP HIV-Syphilis System. This is the first point-of-care lateral-flow multiplex assay cleared for simultaneous detection of HIV-1/2 and Treponema pallidum antibodies from a fingerstick (~10 μL) or plasma. The 15-minute test, used with Chembio’s DPP Micro Reader, enables rapid co-screening of HIV and syphilis, supporting earlier diagnosis and treatment
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.