Global Thymidine Kinase 2 Deficiency Treatment Market
Market Size in USD Billion
CAGR :
% 
 1.31 USD
3.74 USD
2024
2032
1.31 USD
3.74 USD
2024
2032
| 2025 –2032 | |
| 1.31 USD | |
| 3.74 USD | |
|
|
|
|
Thymidine Kinase-2 Deficiency Treatment Market Size
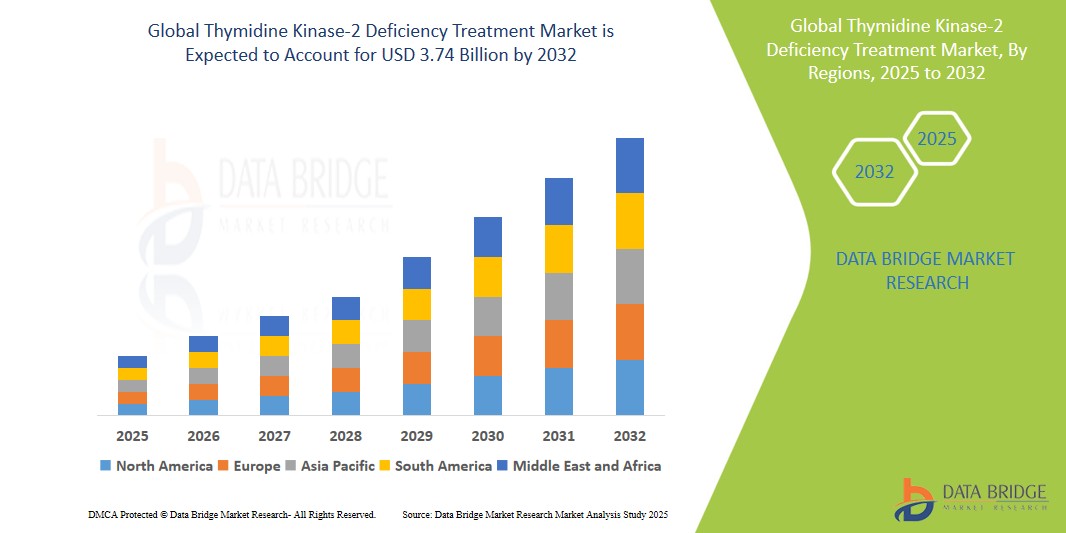
- The global thymidine kinase-2 deficiency treatment market size was valued at USD 1.31 billion in 2024 and is expected to reach USD 3.74 billion by 2032, at a CAGR of 6.10% during the forecast period
- Market growth is largely driven by increasing research and development into rare genetic disorders, advancements in gene therapy and enzyme replacement therapies, and a growing understanding of mitochondrial diseases
- Furthermore, rising patient advocacy for orphan diseases and increasing government initiatives to support the development of therapies for rare conditions are establishing TK2d treatment as a critical area of focus. These converging factors are accelerating the development and uptake of therapeutic solutions, thereby significantly boosting the industry's growth
Gonorrhea Treatment Market Analysis
- Thymidine Kinase-2 Deficiency (TK2d) is a rare, inherited mitochondrial disorder that primarily affects muscle function, leading to progressive muscle weakness (myopathy) and often neurological symptoms. The escalating demand for TK2d Treatment is primarily fueled by improved diagnostic capabilities leading to earlier and more accurate identification of affected individuals, increased awareness among clinicians and patient families, and a growing pipeline of investigational therapies
- North America dominates the thymidine kinase-2 deficiency treatment market with the largest revenue share of 46.31% in 2024, characterized by advanced genetic testing infrastructure, high healthcare expenditure, and a strong presence of key biotechnology and pharmaceutical companies focusing on orphan diseases
- The U.S. is experiencing substantial growth in TK2d Treatment, particularly in specialized rare disease centers and academic medical institutions, driven by research funding for mitochondrial disorders and the establishment of patient registries
- Asia-Pacific is expected to be the fastest-growing region in the thymidine kinase-2 deficiency treatment market during the forecast period due to increasing awareness of genetic disorders, improving diagnostic facilities, and a growing number of clinical trials in countries with large patient populations
- The Nucleoside Therapy segment is expected to dominate the thymidine kinase-2 deficiency treatment market with a market share of 37.54% in 2024, driven by its established reputation as a primary investigational treatment approach aimed at addressing the metabolic deficiency
Report Scope and Gonorrhea Treatment Market Segmentation
|
Attributes |
Gonorrhea Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Thymidine Kinase-2 Deficiency Treatment Market Trends
“Enhanced Therapeutic Approaches Through Gene and Oligonucleotide Therapies”
- A significant and accelerating trend in the global thymidine kinase-2 deficiency treatment market is the deepening integration of advanced therapeutic modalities such as gene therapy and oligonucleotide-based therapies. This fusion of cutting-edge technologies is significantly enhancing the potential for disease modification and improved patient outcomes in this severe mitochondrial disorder
- For instance, adeno-associated virus (AAV) gene therapy vectors are being explored to deliver a functional copy of the TK2 gene to affected cells, aiming to restore enzyme activity and halt disease progression. Similarly, antisense oligonucleotide (ASO) strategies are under investigation to modulate gene expression, potentially addressing the underlying genetic defect
- Gene and oligonucleotide therapies in TK2d Treatment enable features such as highly targeted delivery to specific tissues (e.g., muscle, brain) and providing more intelligent dose optimization based on individual patient response
- For instance, some investigational gene therapies utilize specific viral serotypes to improve cellular uptake over time and can be administered once or infrequently for sustained therapeutic effect. Furthermore, specialized biomarker development and monitoring platforms offer clinicians the ease of tracking treatment efficacy at the molecular level, allowing them to refine therapeutic strategies and ensure optimal patient care. The seamless integration of these advanced therapies into clinical trial designs facilitates centralized control over various aspects of patient management
- Through a single interface, researchers can manage patient data, track safety profiles, and monitor efficacy endpoints, creating a unified and highly efficient drug development workflow. This trend towards more intelligent, precise, and potentially curative treatment options is fundamentally reshaping patient expectations for managing rare genetic disorders like TK2d
- Consequently, companies are developing novel viral vectors and oligonucleotide designs with features such as enhanced tissue specificity and improved safety profiles. The demand for TK2d treatments that offer seamless integration of gene and oligonucleotide approaches is growing rapidly, as rare disease specialists and patient families increasingly prioritize disease-modifying and potentially curative solutions
Thymidine Kinase-2 Deficiency Treatment Market Dynamics
Driver
“Growing Need Due to Advancements in Genetic Diagnostics and Orphan Drug Designations”
- The increasing global understanding and diagnostic capabilities for rare genetic disorders, coupled with the accelerating recognition and support through orphan drug designations for TK2d, are significant drivers for the heightened demand for thymidine kinase-2 deficiency treatment.
- For instance, in April 2024, a leading rare disease foundation highlighted the benefits of early genetic testing for mitochondrial disorders, looking forward to integrating comprehensive newborn screening programs more widely. Such endorsements by key opinion leaders and regulatory bodies are expected to drive the TK2d Treatment industry growth in the forecast period.
- As healthcare providers become more aware of the importance of early diagnosis and the potential for disease-modifying therapies, advanced solutions offer features such as precise genetic confirmation, identification of eligible patients for targeted treatments, and regulatory incentives that accelerate drug development, providing a compelling upgrade over symptomatic-only care.
- the growing popularity of personalized medicine and the desire for improved patient quality of life are making TK2d treatments an integral component of comprehensive rare disease management systems, offering seamless integration with other diagnostic tools and patient support programs.
- The convenience of expedited regulatory pathways for orphan drugs, increased research funding, and the ability to access compassionate use programs are key factors propelling the development and adoption of TK2d treatments. The trend towards evidence-based patient management and the increasing availability of specialized diagnostic panels further contribute to market growth.
Restraint/Challenge
“Concerns Regarding Small Patient Population and High Development Costs”
- Concerns surrounding the extremely small patient population affected by Thymidine Kinase-2 Deficiency, coupled with the relatively high development costs and regulatory complexities associated with orphan drugs and advanced therapies (e.g., gene therapy), pose a significant challenge to broader market penetration
- As TK2d is an ultra-rare disease, the limited number of patients makes it challenging to conduct large-scale clinical trials, leading to extended development timelines and higher per-patient research costs, raising anxieties among pharmaceutical companies about the return on investment
- For instance, reports highlighting the substantial investment required for developing gene therapies for rare diseases have made some companies hesitant to enter this niche market. Addressing these cost concerns through government incentives, venture philanthropy, and risk-sharing models is crucial for encouraging drug development
- Companies in the rare disease space emphasize their collaborations with patient advocacy groups and academic institutions in their marketing to reassure investors. the highly specialized infrastructure and expertise required for administering advanced therapies, along with the long-term follow-up needed for gene therapy patients, can be a barrier to adoption for healthcare facilities, particularly in regions with less developed rare disease care networks or for those with limited resources.
Thymidine Kinase-2 Deficiency Treatment Market Scope
The market is segmented on the basis of treatment type, disease type/severity, end-user, and distribution channel.
By Treatment Type
On the basis of treatment type, the thymidine kinase-2 deficiency treatment market is segmented into nucleoside therapy, gene therapy, enzyme replacement therapy (ERT), symptomatic/supportive care, and Others (e.g., investigational small molecules). The nucleoside therapy segment dominates the largest market revenue share of 37.54% in 2024, driven by its established reputation as a primary investigational treatment approach aimed at addressing the metabolic deficiency by providing substrates for TK2. Healthcare providers often prioritize nucleoside therapy for its potential to replenish mitochondrial DNA precursors and its relatively earlier stage of clinical development compared to other curative options. The market also sees strong demand for nucleoside therapy due to its relatively known mechanism of action and ongoing clinical trials. The gene therapy segment is anticipated to witness the fastest growth rate of 21.7% from 2025 to 2032, fueled by its potential to offer a one-time, disease-modifying, or potentially curative solution by delivering a functional TK2 gene. Gene therapy offers the promise of correcting the underlying genetic defect, and its rapid advancement in rare diseases positions it as a highly anticipated future treatment. The expanding research and investment in AAV-based therapies for neuromuscular disorders also contribute to its rapidly growing popularity.
By Disease Type/Severity
On the basis of disease type/severity, the thymidine kinase-2 deficiency treatment market is segmented into infantile-onset, childhood-onset, adult-onset, and others. The infantile-onset segment held the largest market revenue share in 2024, driven by the severe and rapidly progressive nature of this form of TK2d, which necessitates immediate and intensive therapeutic intervention to improve survival and mitigate neurological damage. The critical need for early diagnosis and treatment for this highly aggressive phenotype drives demand for available therapies. The childhood-onset segment is expected to witness the fastest CAGR from 2025 to 2032, driven by increasing awareness leading to earlier diagnosis of this less rapidly progressive but still debilitating form, allowing for earlier initiation of treatment and potentially better long-term outcomes. The growing number of clinical trials for this age group also contributes to its growth.
By End-User
On the basis of end-user, the thymidine kinase-2 deficiency treatment market is segmented into hospitals, specialty clinics, research & academic institutions, and homecare settings. The hospitals segment accounted for the largest market revenue share in 2024, driven by the increasing number of patients requiring inpatient care for symptom management, complex diagnostic procedures, and administration of specialized treatments, including investigational therapies. Hospitals, especially those with pediatric neurology or genetic departments, serve as primary treatment centers. The research & academic institutions segment is expected to witness the fastest CAGR from 2025 to 2032, driven by their critical role in pioneering new diagnostic methods, conducting preclinical and clinical trials for novel therapies, and advancing the understanding of TK2d pathogenesis. These institutions are at the forefront of innovation and therapeutic development for rare diseases.
By Distribution Channel
On the basis of distribution channel, the thymidine kinase-2 deficiency treatment market is segmented into hospital pharmacies, specialty pharmacies, orphan drug distributors, and online pharmacies. The hospital pharmacies held the largest market revenue share in 2024, driven by the immediate availability of essential medications for inpatient care and the specialized handling required for some investigational or infused therapies. Hospital pharmacies play a crucial role in ensuring access and proper administration of complex treatments. The orphan drug distributors segment is expected to witness the fastest CAGR from 2025 to 2032, primarily due to their specialized logistics and expertise in managing the complex supply chain for rare disease drugs, ensuring these high-value, often temperature-sensitive therapies reach the limited patient population efficiently. Their direct collaboration with manufacturers and specialized centers drives this segment's growth.
Thymidine Kinase-2 Deficiency Treatment Market Regional Analysis
- North America dominates the thymidine kinase-2 deficiency treatment market with the largest revenue share of 46.31% in 2024, driven by advanced genetic testing capabilities, significant funding for rare disease research, and a robust regulatory framework that supports orphan drug development
- The region benefits from a high concentration of specialized medical centers with expertise in mitochondrial disorders, strong patient advocacy groups, and active participation in global clinical trials for TK2d
- This widespread adoption is further supported by high healthcare expenditure and a proactive approach to rare disease identification and management
U.S. Thymidine Kinase-2 Deficiency Treatment Market Insight
The U.S. thymidine kinase-2 deficiency treatment market captured the largest revenue share of 81% within North America in 2024, fueled by leading-edge genetic research, the presence of major biotechnology and pharmaceutical companies investing in rare diseases, and robust patient support networks. The strong emphasis on personalized medicine and genetic screening, coupled with substantial government and private funding for orphan disease R&D, further propels the market.
Europe Thymidine Kinase-2 Deficiency Treatment Market Insight
The European thymidine kinase-2 deficiency treatment market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by increasing awareness of rare genetic disorders, advancements in diagnostic techniques, and supportive regulatory frameworks for orphan drugs across several European countries. Collaborative research initiatives and the establishment of rare disease networks further contribute to market growth.
U.K. Thymidine Kinase-2 Deficiency Treatment Market Insight
The U.K. thymidine kinase-2 deficiency treatment market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by a strong research base in genetics and rare diseases, active patient advocacy, and the National Health Service's (NHS) commitment to providing specialized care for complex conditions. Increasing access to genetic testing and participation in international clinical trials also contribute to growth.
Germany Thymidine Kinase-2 Deficiency Treatment Market Insight
The German thymidine kinase-2 deficiency treatment market is expected to expand at a considerable CAGR during the forecast period, fueled by its robust healthcare infrastructure, leading genetic research institutions, and a strong emphasis on clinical research for rare diseases. Germany's advanced diagnostic capabilities and specialized treatment centers position it as a key market for TK2d therapies.
Asia-Pacific Thymidine Kinase-2 Deficiency Treatment Market Insight
The Asia-Pacific thymidine kinase-2 deficiency treatment market is poised to grow at the fastest CAGR of over 24% in 2024, driven by increasing awareness and diagnostic capabilities for genetic disorders, growing investments in healthcare infrastructure, and rising clinical trial activity in countries like Japan, China, and South Korea. The development of rare disease policies and improving access to specialized medical care also contribute to regional growth.
Japan Thymidine Kinase-2 Deficiency Treatment Market Insight
The Japan thymidine kinase-2 deficiency treatment market is gaining momentum due to its advanced medical research capabilities, high adoption rate of genomic medicine, and increasing focus on orphan drug development. The Japanese market benefits from a well-established healthcare system and a growing understanding of mitochondrial diseases, driving demand for innovative therapies.
China Thymidine Kinase-2 Deficiency Treatment Market Insight
The China thymidine kinase-2 deficiency treatment market accounted for the largest market revenue share in Asia Pacific in 2024, attributed to its large population, increasing investments in healthcare infrastructure, and emerging capabilities in genetic diagnostics and gene therapy research. Growing awareness of rare diseases and government support for developing treatments for unmet medical needs are key factors propelling the market in China.
Thymidine Kinase-2 Deficiency Treatment Market Share
Thymidine kinase-2 deficiency treatment market industry is primarily led by well-established companies, including:
- Genentech, Inc. (U.S.)
- Recursion Pharmaceuticals (U.S.)
- Ultragenyx Pharmaceutical Inc. (U.S.
- Stealth BioTherapeutics (U.S.)
- Alexion Pharmaceuticals, Inc. (AstraZeneca) (U.S.)
- Taked Pharmaceutical Company Limited (Japan
- Sarepta Therapeutics, Inc. (U.S.)
- Rocket Pharmaceuticals, Inc. (U.S.)
- Asklepios BioPharmaceutical, Inc. (AskBio - Bayer) (U.S.)
- BridgeBio Pharma, Inc. (U.S.)
- Neurocrine Biosciences, Inc. (U.S.)
- PTC Therapeutics, Inc. (U.S.)
- Chiesi Farmaceutici S.p.A. (Italy)
- Audentes Therapeutics (Astellas Pharma) (U.S.)
- Ionis Pharmaceuticals, Inc. (U.S.)
- Amicus Therapeutics (U.S.)
Latest Developments in Global Thymidine Kinase-2 Deficiency Treatment Market
- In April 2023, Ultragenyx Pharmaceutical Inc., a company focused on rare and ultra-rare diseases, announced positive interim data from its ongoing clinical trial of an investigational nucleoside therapy for Thymidine Kinase-2 Deficiency
- In March 2023, Stealth BioTherapeutics, a clinical-stage biotechnology company, initiated a new Phase 2 study for its investigational mitochondrial therapy in patients with TK2d, expanding its clinical program and demonstrating continued commitment to addressing this rare genetic disorder
- In March 2023, Rocket Pharmaceuticals, Inc., a leading gene therapy company, presented preclinical data for its investigational gene therapy for TK2d at a major rare disease conference, highlighting promising results in animal models
- In February 2023, a collaboration between a leading academic research institution and BridgeBio Pharma, Inc., a company focused on genetic diseases, received new grant funding to accelerate preclinical research into novel therapeutic targets for TK2d
- In January 2023, Alexion Pharmaceuticals, Inc. (AstraZeneca) announced the expansion of its rare disease diagnostic support program to include broader genetic screening for mitochondrial disorders, which could lead to earlier identification of TK2d patients
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.