Global Tralokinumab Market
Market Size in USD Million
CAGR :
% 
 USD
500.50 Million
USD
1,275.07 Million
2024
2032
USD
500.50 Million
USD
1,275.07 Million
2024
2032
| 2025 –2032 | |
| USD 500.50 Million | |
| USD 1,275.07 Million | |
|
|
|
|
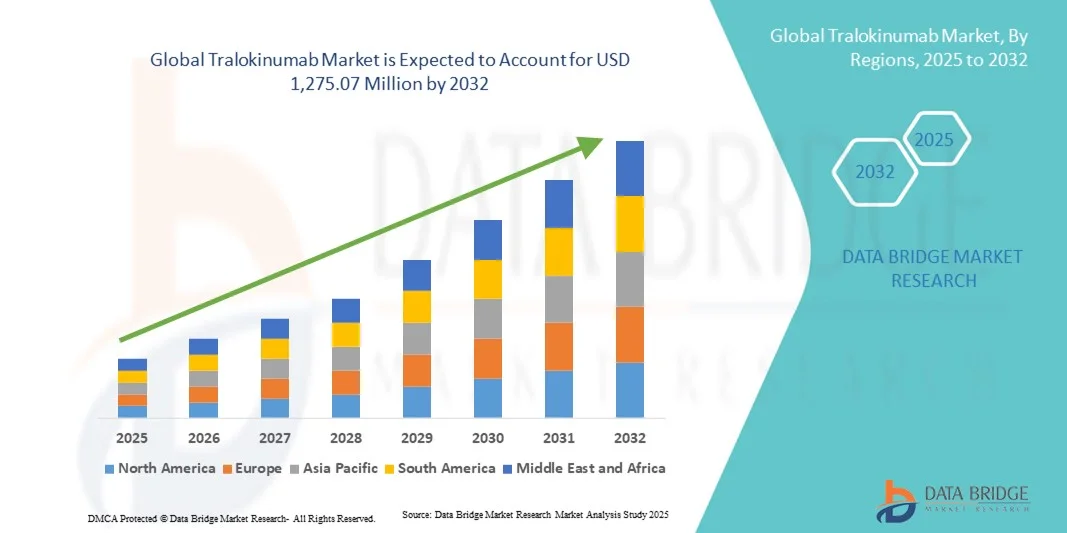
Tralokinumab Market Size
- The global Tralokinumab market size was valued at USD 500.50 million in 2024 and is expected to reach USD 1,275.07 million by 2032, at a CAGR of 12.40% during the forecast period
- The market growth is largely fueled by the growing prevalence of inflammatory skin conditions and increased adoption of targeted biologic therapies such as Tralokinumab, which is a humanised monoclonal antibody targeting IL‑13
- Furthermore, rising demand for effective, safe and well‑tolerated biologic treatments in dermatology and the expansion of reimbursement and awareness in key markets are establishing Tralokinumab as a modern therapy choice. These converging factors are accelerating the uptake of Tralokinumab, thereby significantly boosting the industry’s growth
Tralokinumab Market Analysis
- Tralokinumab, a monoclonal antibody targeting IL 13, is increasingly vital in the treatment of moderate-to-severe atopic dermatitis and other inflammatory conditions due to its targeted mechanism, favorable safety profile, and potential for long-term disease management
- The escalating demand for Tralokinumab is primarily fueled by the rising prevalence of atopic dermatitis, increasing adoption of biologic therapies, and growing awareness among healthcare providers and patients regarding effective, targeted treatment options
- North America dominated the Tralokinumab market with the largest revenue share of 43% in 2024, characterized by well-established healthcare infrastructure, high adoption of biologics, and a strong presence of key pharmaceutical players, with the U.S. experiencing substantial growth driven by regulatory approvals, reimbursement support, and ongoing clinical research into IL 13 targeted therapies
- Asia-Pacific is expected to be the fastest-growing region in the Tralokinumab market during the forecast period due to increasing healthcare access, rising prevalence of atopic dermatitis, and expanding investment in biologic therapies in emerging markets
- Atopic dermatitis segment dominated the Tralokinumab market with a market share of 65.8% in 2024, driven by Tralokinumab’s approval for moderate-to-severe cases and its growing adoption as a preferred therapy option among dermatologists and patients
Report Scope and Tralokinumab Market Segmentation
|
Attributes |
Tralokinumab Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework |
Tralokinumab Market Trends
Expansion of Targeted Biologics and Personalized Therapy
- A significant and accelerating trend in the global Tralokinumab market is the growing focus on targeted biologics and personalized treatment approaches for moderate-to-severe atopic dermatitis, offering more effective disease management with fewer side effects
- For instance, Tralokinumab selectively inhibits IL‑13, providing tailored therapy for patients with Th2-driven inflammation, positioning it as a preferred alternative to broad immunosuppressive treatments
- Integration of Tralokinumab into combination therapy regimens with topical corticosteroids and emollients allows dermatologists to optimize patient outcomes while reducing systemic exposure, enhancing overall treatment efficacy
- Biomarker-driven patient stratification is gaining traction, enabling healthcare providers to identify individuals most such likely to respond to Tralokinumab, thus improving clinical outcomes and patient satisfaction
- This trend towards precision medicine and biologic-targeted therapy is reshaping clinical practice in dermatology, with pharmaceutical companies such as AstraZeneca developing next-generation IL‑13 inhibitors and exploring expanded indications
- The demand for biologics that offer targeted efficacy, reduced side effects, and personalized treatment plans is growing rapidly across both developed and emerging markets, as patient and physician awareness of advanced therapies increases
Tralokinumab Market Dynamics
Driver
Rising Prevalence of Atopic Dermatitis and Adoption of Biologic Therapies
- The increasing prevalence of moderate-to-severe atopic dermatitis, coupled with rising adoption of biologic therapies, is a significant driver of Tralokinumab demand globally
- For instance, in 2024, AstraZeneca reported expanded clinical adoption of Tralokinumab across North America and Europe, reflecting growing physician confidence in its efficacy and safety profile
- As healthcare providers seek more effective long-term management options for chronic skin conditions, Tralokinumab offers advantages such as targeted IL‑13 inhibition, predictable dosing, and improved patient adherence
- Furthermore, reimbursement support and increasing insurance coverage for biologic treatments are encouraging broader adoption among patients who previously relied on conventional therapies
- The convenience of subcutaneous administration, combined with favorable tolerability, enhances patient compliance and positions Tralokinumab as a preferred therapy for moderate-to-severe cases
- Ongoing awareness campaigns and education for dermatologists and patients on the benefits of biologics further support the growing uptake of Tralokinumab across key markets
Restraint/Challenge
High Treatment Costs and Limited Long-Term Real-World Data
- The high cost of Tralokinumab therapy compared to conventional treatments presents a barrier to adoption, particularly in price-sensitive regions and emerging markets
- For instance, the annual treatment cost for biologic therapy may limit patient access despite proven clinical efficacy, restricting market growth in certain demographics
- In addition, limited long-term real-world safety and efficacy data can create hesitancy among healthcare providers when prescribing Tralokinumab to broader patient populations
- Monitoring for adverse events, including rare immunological reactions, remains critical, requiring physicians and patients to weigh benefits against potential risks carefully
- While insurance coverage is improving in some regions, out-of-pocket expenses for biologics remain a concern, potentially slowing uptake in cost-conscious populations
- Overcoming these challenges through patient assistance programs, expanding long-term clinical evidence, and strategic pricing will be essential for sustained market growth globally
Tralokinumab Market Scope
The market is segmented on the basis of type, application, end‑users and distribution channel
- By Type
On the basis of type, the Tralokinumab market is segmented into anti-inflammatories, antiasthmatics, antifibrotics, monoclonal antibodies, and skin disorder therapies. The monoclonal antibodies segment dominated the market with the largest market revenue share in 2024, driven by Tralokinumab being a targeted IL‑13 inhibitor widely adopted for moderate-to-severe atopic dermatitis. Healthcare providers often prioritize monoclonal antibodies due to their high efficacy, targeted mechanism, and favorable safety profile. The market sees strong demand because monoclonal antibodies are increasingly preferred over broad immunosuppressive therapies. Manufacturing and distribution networks for biologics are well-established, supporting rapid uptake. Patients benefit from predictable dosing and lower systemic side effects, reinforcing adoption. Pharmaceutical companies continue to invest heavily in this segment due to its revenue potential and robust clinical evidence.
The skin disorder therapies segment is expected to witness the fastest growth rate from 2025 to 2032, fueled by expanding Tralokinumab adoption in dermatology. Patients and dermatologists increasingly prefer targeted biologics for moderate-to-severe atopic dermatitis. Ongoing clinical trials and real-world evidence support its effectiveness, accelerating market growth. Expansion into adolescent and pediatric populations is contributing to faster uptake. Awareness campaigns and physician education further drive adoption. Emerging markets with growing access to advanced therapies present additional growth opportunities.
- By Application
On the basis of application, the Tralokinumab market is segmented into atopic dermatitis, asthma, and others. The atopic dermatitis segment dominated the market in 2024 with a market share of 65.8%, driven by Tralokinumab’s approval for moderate-to-severe AD and increasing adoption among dermatologists. High global prevalence of AD ensures a large patient base for targeted biologics. Physicians prefer Tralokinumab due to specific IL‑13 inhibition and lower systemic side effects. Strong clinical trial support and growing insurance coverage in developed markets bolster the segment. Patient adherence is enhanced by subcutaneous administration and manageable dosing schedules. Marketing efforts and patient awareness initiatives further reinforce dominance.
The asthma segment is expected to witness the fastest growth from 2025 to 2032, driven by ongoing investigations of Tralokinumab for IL‑13 mediated asthma and allergic conditions. Pipeline expansion into respiratory indications creates strong future growth potential. Regulatory approvals for asthma treatment would further broaden the patient base. Adoption in emerging markets with increasing asthma prevalence supports faster growth. Real-world evidence of IL‑13 inhibition benefits strengthens physician interest. The segment benefits from unmet medical need for targeted biologic therapies in asthma care.
- By End-Users
On the basis of end-users, the Tralokinumab market is segmented into clinic, hospital, and others. The hospital segment dominated in 2024, as administration and monitoring of Tralokinumab require specialized medical oversight. Hospitals provide infrastructure for biologic therapy storage, dosing, and patient monitoring. Clinicians prefer hospitals to manage adverse events and ensure treatment adherence. Large hospitals and tertiary care centers capture most revenue due to higher patient throughput. Hospital pharmacies facilitate controlled distribution and reimbursement processes. Relationships between pharmaceutical companies and hospitals reinforce this segment’s dominance.
The clinic segment is expected to witness the fastest growth from 2025 to 2032, driven by adoption of Tralokinumab in outpatient dermatology and allergy clinics. Shift toward subcutaneous administration allows patients to receive therapy outside hospital settings. Expansion in private clinics and specialty centers supports faster uptake. Physician familiarity and patient convenience drive clinic adoption. Emerging markets are promoting outpatient biologic therapy to reduce hospital burden. Home-based administration and telemedicine support further accelerate growth.
- By Distribution Channel
On the basis of distribution channel, the Tralokinumab market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The hospital pharmacy segment dominated in 2024 due to centralized dispensing of high-cost biologics such as Tralokinumab. Hospitals ensure proper storage, manage inventory, and monitor patient adherence. Reimbursement mechanisms are tied to hospital pharmacy distribution. Physicians and patients prefer hospital pharmacies for initial prescription fulfillment. Established supply chains support consistent availability and therapy continuity. Integration with hospital electronic medical records reinforces dominance.
The online pharmacy segment is expected to witness the fastest growth from 2025 to 2032, fueled by healthcare digitalization and rising e-pharmacy adoption. Patients increasingly prefer home delivery of prescribed biologics for convenience and adherence. Telehealth consultations and e-prescriptions support online fulfillment. Growth is rapid in developed markets with supportive regulations. Online channels improve access in remote or underserved areas. Convenience-driven consumer behavior and growing awareness of Tralokinumab further drive growth.
Tralokinumab Market Regional Analysis
- North America dominated the Tralokinumab market with the largest revenue share of 43% in 2024, characterized by well-established healthcare infrastructure, high adoption of biologics, and a strong presence of key pharmaceutical players, with the U.S. experiencing substantial growth driven by regulatory approvals, reimbursement support, and ongoing clinical research into IL 13 targeted therapies
- Patients and healthcare providers in the region highly value the targeted efficacy, favorable safety profile, and predictable dosing schedule offered by Tralokinumab, making it a preferred treatment for dermatologists
- This widespread adoption is further supported by well-established healthcare infrastructure, strong insurance coverage and reimbursement policies, and growing awareness among clinicians and patients, establishing Tralokinumab as a leading therapy for atopic dermatitis and other IL‑13 mediated conditions
U.S. Tralokinumab Market Insight
The U.S. Tralokinumab market captured the largest revenue share of 78% in 2024 within North America, fueled by the high prevalence of moderate-to-severe atopic dermatitis and increasing adoption of biologic therapies. Patients and healthcare providers are prioritizing targeted treatments with improved safety profiles and predictable dosing schedules. Growing awareness among dermatologists regarding IL‑13 inhibition and clinical trial evidence further propels market adoption. In addition, robust insurance coverage and reimbursement policies enhance accessibility to Tralokinumab for patients. Expansion of outpatient dermatology and allergy clinics also supports increased prescription and usage. The integration of Tralokinumab into standard care pathways for atopic dermatitis is significantly contributing to market growth.
Europe Tralokinumab Market Insight
The Europe Tralokinumab market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by rising prevalence of atopic dermatitis and growing acceptance of biologic therapies. Increasing awareness among healthcare professionals about targeted IL‑13 inhibitors is encouraging adoption. Government initiatives and favorable reimbursement policies across countries such as Germany and France are facilitating broader market penetration. The region is witnessing growth in both hospital and clinic-based therapy administration. Patients are also seeking advanced, long-term management options, supporting demand. Real-world evidence and clinical guidelines promoting Tralokinumab use in dermatology contribute to market expansion.
U.K. Tralokinumab Market Insight
The U.K. Tralokinumab market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by rising awareness of biologics and increased prevalence of moderate-to-severe atopic dermatitis. Patients and clinicians are increasingly preferring targeted therapies over conventional systemic treatments. The presence of a strong healthcare infrastructure and favorable insurance coverage supports broader adoption. Physician familiarity and ongoing education regarding Tralokinumab reinforce confidence in prescribing. The integration of Tralokinumab into outpatient clinic workflows enhances convenience and patient adherence. Growing patient demand for safe and effective long-term management options is expected to continue driving growth.
Germany Tralokinumab Market Insight
The Germany Tralokinumab market is expected to expand at a considerable CAGR during the forecast period, fueled by increasing prevalence of atopic dermatitis and awareness of advanced biologic therapies. Strong healthcare infrastructure and structured reimbursement programs enable widespread adoption. Dermatologists and specialty clinics are actively prescribing Tralokinumab for moderate-to-severe cases. Emphasis on targeted therapies and precision medicine aligns with the country’s focus on innovative healthcare solutions. Hospital and clinic-based administration channels further strengthen market presence. Real-world evidence supporting long-term efficacy enhances physician and patient confidence in therapy adoption.
Asia-Pacific Tralokinumab Market Insight
The Asia-Pacific Tralokinumab market is poised to grow at the fastest CAGR of 22% during the forecast period of 2025 to 2032, driven by rising prevalence of atopic dermatitis, growing healthcare access, and expanding adoption of biologic therapies in emerging economies. Increased awareness among dermatologists and patients supports rapid market penetration. Governments are promoting digital health initiatives, improving therapy accessibility. Expansion of outpatient clinics and specialty centers contributes to growing usage. Increasing healthcare expenditure and insurance coverage further facilitate adoption. Rising interest in advanced and targeted therapies in countries such as China, India, and Japan accelerates market growth.
Japan Tralokinumab Market Insight
The Japan Tralokinumab market is gaining momentum due to high prevalence of atopic dermatitis, increasing healthcare infrastructure, and a strong focus on advanced biologic treatments. Patients and physicians are adopting targeted IL‑13 inhibitors for better efficacy and safety. Integration into clinic-based and hospital-based treatment pathways supports therapy continuity. Real-world data and ongoing clinical trials reinforce physician confidence. An aging population and demand for long-term disease management are driving adoption. Government and private healthcare initiatives promoting access to innovative biologics further contribute to market expansion.
India Tralokinumab Market Insight
The India Tralokinumab market accounted for the largest revenue share in Asia-Pacific in 2024, attributed to increasing prevalence of moderate-to-severe atopic dermatitis and rising healthcare awareness. The expanding middle class and improving access to dermatology clinics support growing adoption. Availability of biologic therapies, coupled with government initiatives to promote specialty care, is boosting market growth. Patient awareness and physician education on targeted therapies further reinforce demand. Emerging insurance coverage for biologics facilitates therapy access. Overall, affordability, accessibility, and increasing clinician familiarity are key factors propelling the Tralokinumab market in India.
Tralokinumab Market Share
The Tralokinumab industry is primarily led by well-established companies, including:
- Leo Pharma A/S (Denmark)
- AstraZeneca (U.K.)
- AbbVie Inc. (U.S.)
- Amgen Inc. (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- Novartis AG (Switzerland)
- Sanofi (France)
- Pfizer Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- Bayer AG (Germany)
- Boehringer Ingelheim International GmbH.(Germany)
- Regeneron Pharmaceuticals Inc (U.S.)
- Eli Lilly and Company (U.S.)
- BioNTech SE (Germany)
- Vertex Pharmaceuticals (U.S.)
- GSK plc (U.K.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Mitsubishi Tanabe Pharma Corporation (Japan)
- Sandoz Group AG (Germany)
What are the Recent Developments in Global Tralokinumab Market?
- In September 2025, at the European Academy of Dermatology and Venereology (EADV) Congress, LEO Pharma presented late‑breaking data indicating that a response to tralokinumab at Week 16 predicts long‑term (up to three years) stability of treatment outcomes in moderate‑to‑severe AD
- In July 2025, LEO Pharma announced positive 16‑week interim results from the Phase 3b ADHAND trial of tralokinumab in adult patients with moderate‐to‐severe atopic dermatitis localized to the hands. The trial showed that patients treated with tralokinumab 300 mg every 2 weeks achieved statistically significant improvements (IGA–AHE 0/1) vs placebo by Week 16
- In June 2024, the U.S. Food & Drug Administration (FDA) approved a new 300 mg single‑dose autoinjector formulation of Tralokinumab for adult patients with moderate‑to‑severe atopic dermatitis (AD), offering a more convenient self‑administration option
- In June 2024, the FDA approved a single‑dose autoinjector formulation of tralokinumab‑ldrm (Adbry) for adults with moderate‑to‑severe atopic dermatitis. The approval underscores patient‑centric innovation, focusing on ease of self‑administration and potentially improving adherence and satisfaction
- In December 2023, the Food & Drug Administration (FDA) approved Adbry® (tralokinumab ldrm) for pediatric patients aged 12 17 years with moderate to severe atopic dermatitis (AD) whose disease is inadequately controlled by topical therapies
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.