Global Traumatic Brain Injury Drugs Market
Market Size in USD Million
CAGR :
% 
 USD
618.09 Million
USD
978.28 Million
2024
2032
USD
618.09 Million
USD
978.28 Million
2024
2032
| 2025 –2032 | |
| USD 618.09 Million | |
| USD 978.28 Million | |
|
|
|
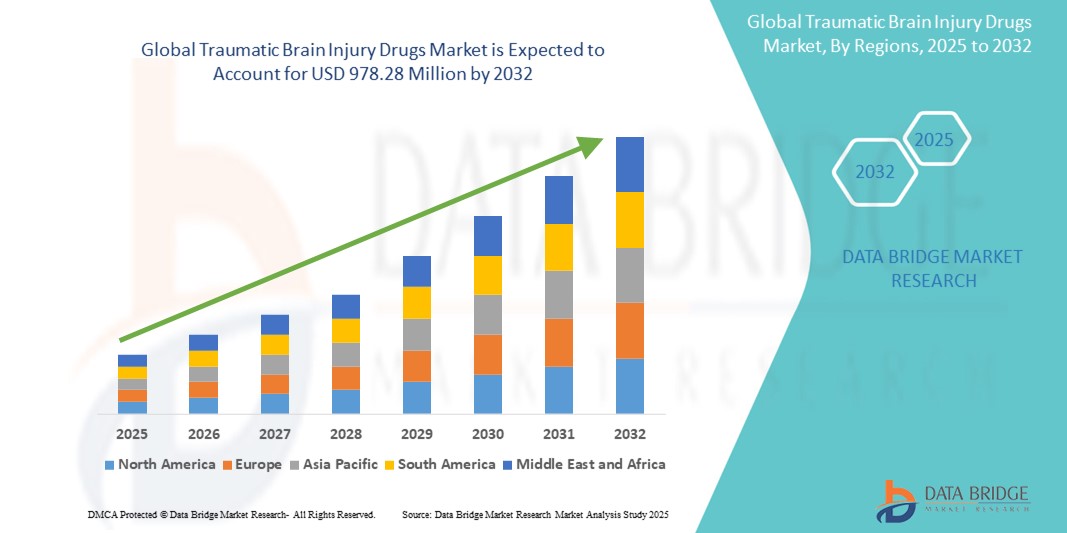
Traumatic Brain Injury Drugs Market Size
- The global traumatic brain injury drugs market was valued at USD 618.09 million in 2024 and is expected to reach USD 978.28 million by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 5.91%, primarily driven by the primarily driven by rising traumatic brain injury incidence
- This growth is driven by factors such as increasing focus on early diagnosis and intervention and increasing incidence of road accidents and sports injuries
Traumatic Brain Injury Drugs Market Analysis
- Traumatic brain injury drugs are medications used to manage symptoms and complications associated with brain injuries caused by external mechanical force. These drugs aim to reduce inflammation, prevent seizures, manage pain, and improve neurological function following a traumatic brain injury
- The global traumatic brain injury drugs market is witnessing significant growth, projected to expand at a compound annual growth rate of 8.7 percent in the coming years. This growth is primarily driven by the increasing incidence of traumatic brain injuries worldwide, fueled by rising cases of road accidents, sports injuries, falls, and combat-related trauma. In addition, growing awareness about early intervention and improved healthcare infrastructure are further accelerating market expansion
- Key drug classes in this market include anti-seizure medications, stimulants, anticoagulants, and pain relievers. Among these, anti-seizure drugs hold a considerable share due to their effectiveness in preventing post-traumatic seizures, a common complication of brain injuries. The injectable route of administration dominates the market, given its rapid efficacy in emergency care settings
- In March 2025, the U.S. Food and Drug Administration approved a new neuroprotective therapy aimed at improving recovery outcomes for patients with traumatic brain injuries, marking a significant advancement in the field of traumatic brain injury drugs
- The growing adoption of these treatments in both hospital and homecare settings highlights the increasing emphasis on comprehensive patient management. North America currently leads the market, but Asia-Pacific is expected to witness the fastest growth, supported by healthcare advancements and a rising patient pool
Report Scope and Traumatic Brain Injury Drugs Market Segmentation
|
Attributes |
Traumatic Brain Injury Drugs Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Traumatic Brain Injury Drugs Market Trends
“Growing Focus on Neuroprotective Therapies”
- One prominent trend shaping the global traumatic brain injury drugs market is the growing focus on neuroprotective therapies. Traditionally, treatments for traumatic brain injury have been limited to managing symptoms such as seizures, pain, and inflammation. However, recent advancements are shifting attention toward drugs that can actively protect brain tissue and promote neural regeneration
- In early 2025, several pharmaceutical companies initiated clinical trials for innovative compounds aimed at minimizing secondary brain damage and improving long-term cognitive outcomes
- This trend is fueled by the increasing understanding of the complex pathophysiology of traumatic brain injuries and the urgent need for more effective interventions. Moreover, supportive regulatory policies and rising healthcare investments globally are accelerating research and development in this area
- As a result, neuroprotective therapies are expected to play a pivotal role in reshaping treatment landscapes, offering hope for improved recovery rates among patients suffering from traumatic brain injuries
Traumatic Brain Injury Drugs Market Dynamics
Driver
“Increasing Focus on Early Diagnosis and Intervention”
- The increasing focus on early diagnosis and intervention for traumatic brain injuries is significantly contributing to the rising demand for effective drug therapies
- Healthcare systems worldwide are emphasizing prompt treatment of brain injuries to prevent complications such as brain swelling, seizures, and long-term cognitive decline
- Early administration of drugs, particularly neuroprotective agents and anti-inflammatory medications, has been shown to improve recovery rates and reduce mortality in traumatic brain injury patients
- Advancements in diagnostic imaging and monitoring technologies, such as MRI and CT scans, enable quicker identification of traumatic brain injuries, supporting timely pharmaceutical intervention
- As medical practitioners become more aware of the benefits of early treatment, the demand for targeted traumatic brain injury drugs continues to grow, driving market expansion
For instance,
- In March 2025, an article by the Brain Injury Association reported that early treatment protocols, including immediate administration of neuroprotective medications, reduced severe disability rates by over 30 percent in traumatic brain injury patients
- In October 2024, a study published by the Journal of Neurotrauma emphasized that rapid diagnosis and treatment significantly improve recovery outcomes, reinforcing the need for readily available pharmaceutical solutions for traumatic brain injuries
- As a result of the growing emphasis on early diagnosis and intervention, there is a significant increase in the demand for effective traumatic brain injury drugs to ensure better patient outcomes and faster recovery
Opportunity
“Growing Integration of Advanced Biomarkers and Personalized Medicine”
- The growing integration of advanced biomarkers and personalized medicine offers significant opportunities for the development of targeted traumatic brain injury drug therapies
- Biomarker-based approaches enable clinicians to accurately assess the severity of brain injuries and predict patient outcomes, paving the way for personalized treatment plans
- By identifying specific biological indicators associated with traumatic brain injury, pharmaceutical companies can develop more effective and tailored drug therapies that address individual patient needs
For instance,
- In February 2025, according to an article published in The Lancet Neurology, emerging biomarkers such as glial fibrillary acidic protein (GFAP) and ubiquitin carboxyl-terminal hydrolase L1 (UCH-L1) have demonstrated high accuracy in detecting traumatic brain injuries, enabling timely and personalized therapeutic interventions
- In October 2024, a study published in the Journal of Neurotrauma highlighted that personalized medicine approaches, which incorporate individual biomarker profiles, can significantly enhance treatment efficacy and reduce adverse effects in traumatic brain injury patients
- The utilization of advanced biomarkers and personalized medicine strategies in traumatic brain injury management can lead to improved treatment outcomes, reduced complications, and enhanced patient recovery experiences
Restraint/Challenge
“High Cost of Developing Traumatic Brain Injury Drugs”
- The high cost of developing traumatic brain injury drugs presents a significant challenge for the market, affecting both pharmaceutical companies and healthcare systems
- Clinical trials for traumatic brain injury treatments require substantial investment due to the complexity of brain injury mechanisms, long follow-up periods, and the need for advanced diagnostic tools
- This significant financial burden often discourages smaller biotech firms and even larger pharmaceutical companies from investing in new drug development, limiting the pipeline of innovative therapies
For instance,
- In January 2025, according to an article published in The Journal of Head Trauma Rehabilitation, the estimated cost of bringing a single traumatic brain injury drug to market exceeds USD 1.5 billion, primarily due to extensive clinical trials and regulatory requirements. These high costs act as a deterrent for companies considering investment in this therapeutic area
- As a result, the limited number of active clinical programs and high development expenses restrict the availability of advanced treatment options, posing a substantial hurdle to market growth and patient access to novel therapies
Traumatic Brain Injury Drugs Market Scope
The market is segmented on the basis of drug and end user.
|
Segmentation |
Sub-Segmentation |
|
By Drug |
|
|
By End-User |
|
Traumatic Brain Injury Drugs Market Regional Analysis
North America is the Dominant Region in the Traumatic Brain Injury Drugs Market”
- North America is the dominant region in the global traumatic brain injury drugs market, driven by robust healthcare infrastructure, high patient awareness, and significant investments in neuroscience research and development
- The region experiences a high prevalence of traumatic brain injuries, largely due to sports-related injuries, road accidents, and falls among the elderly population, creating a sustained demand for effective treatment options. Moreover, strong support from government bodies and favorable reimbursement frameworks encourage pharmaceutical advancements and the availability of novel therapies
- For instance, in March 2024, the U.S. National Institutes of Health (NIH) announced increased funding for brain injury research, further cementing the region’s leadership in this field
- Within North America, the U.S. holds the largest share, owing to its advanced healthcare facilities, extensive clinical trials, and presence of major market players, making it the primary contributor to the region's dominance in the traumatic brain injury drugs market
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- Asia-Pacific is projected to register the highest growth rate in the global traumatic brain injury drugs market, fueled by rising healthcare expenditure, improving access to advanced treatments, and increasing awareness about traumatic brain injuries
- Rapid urbanization and growing incidences of road traffic accidents and sports-related injuries are contributing significantly to the rising patient pool in the region. Furthermore, governments across countries such as China, India, and Japan are actively investing in healthcare infrastructure and neurological research, thereby enhancing the availability and affordability of treatment options
- The expanding pharmaceutical sector and growing collaborations between local and global pharmaceutical companies are expected to drive further innovation and market expansion
- Notably, China is anticipated to witness the highest compound annual growth rate (CAGR) within the Asia-Pacific region, supported by its large population base, increasing healthcare reforms, and strategic focus on neurological disease management and drug development
Traumatic Brain Injury Drugs Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Astrocyte Pharmaceuticals Inc. (U.S.)
- BIOMÉRIEUX (France)
- BrainScope Company Inc (U.S.)
- Hoth Therapeutics (U.S.)
- Ischemix (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- Medicortex Finland Oyj (Plc) (Finland)
- Oxeia Biopharmceuticals (U.S.)
- Oculogica (U.S.)
- NeuraSignal, Inc. (U.S.)
- Neuren Pharmaceuticals (Australia)
- QuesGen Systems, Inc. (U.S.)
- SanBio Co,Ltd. (Japan)
- Stemedica Cell Technologies, Inc. (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Tiziana Life Sciences (U.K.)
Latest Developments in Global Traumatic Brain Injury Drugs Market
- In February 2025, Tiziana Life Sciences, Ltd. announced the publication of a groundbreaking study in Nature Neuroscience, which revealed that nasal delivery of Tiziana’s anti-CD3 monoclonal antibody notably decreased neuroinflammation and enhanced recovery outcomes
- In July 2024, SanBio Co., Ltd. announced that it had received conditional and time-limited marketing approval in Japan for its human somatic stem cell-processed product, “AKUUGO suspension for intracranial implantation”. The approval covers the use of AKUUGO for improving chronic motor paralysis caused by traumatic brain injury
- In August 2023, Astrocyte Pharmaceuticals Inc. announced the successful completion of an oversubscribed pre-Series B Bridge financing round, raising over USD 6 million. The round was led by Dreavent Capital, with participation from the Alzheimer’s Drug Discovery Foundation and other investors. This funding will support the accelerated clinical development of AST-004, a promising therapeutic candidate targeting a wide range of acute brain injuries, including stroke and traumatic brain injury. In addition, the investment will enable further exploration of AST-004’s potential in preclinical models for Alzheimer’s disease
- In September 2022, Hoth Therapeutics, Inc. announced that it had entered into an agreement with Altasciences, a fully integrated early-phase contract research and manufacturing organization, for the development and production of the HT-TBI drug product formulation. HT-TBI is being developed as an innovative, point-of-care treatment aimed at managing and potentially preventing secondary brain injuries, such as brain swelling and inflammation, associated with ischemic stroke and traumatic brain injury
- In July 2021, Ischemix, Inc. announced that the United States Department of Defense awarded the company 2.9 million dollars to support a Phase 1 clinical study of CMX-2043, an innovative compound designed for the treatment of acute traumatic brain injury. CMX-2043 is a multi-modal, cytoprotective compound derived from a naturally occurring molecule found in the human body. It has previously shown promising safety and effectiveness in preclinical models of traumatic brain injury
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.