Global Ultra Rare Pediatric Cancer Drug Market
Market Size in USD Million
CAGR :
% 
 USD
122.24 Million
USD
223.09 Million
2024
2032
USD
122.24 Million
USD
223.09 Million
2024
2032
| 2025 –2032 | |
| USD 122.24 Million | |
| USD 223.09 Million | |
|
|
|
|
Ultra-Rare Pediatric Cancer Drug Market Size
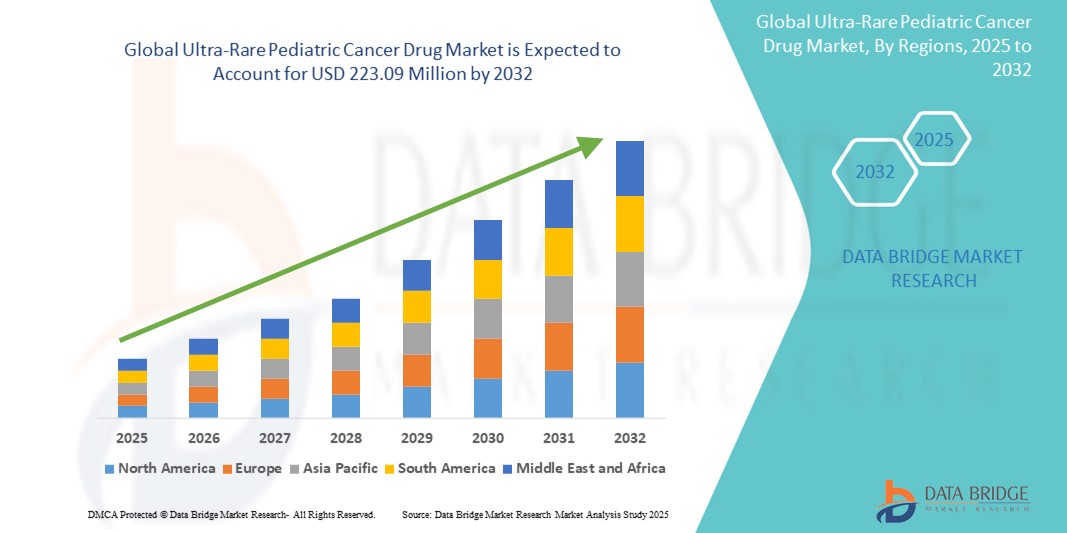
- The global ultra-rare pediatric cancer drug market size was valued at USD 122.24 million in 2024 and is expected to reach USD 223.09 million by 2032, at a CAGR of 7.81% during the forecast period
- The market growth is largely driven by increasing research focus, government incentives, and regulatory support for orphan and pediatric drug development, leading to an expansion in life-saving therapies for ultra-rare pediatric malignancies
- Furthermore, rising awareness, improved genetic profiling, and enhanced diagnostic capabilities are driving early detection and targeted treatment, establishing ultra-rare pediatric cancer drugs as a critical segment in precision oncology. These converging factors are accelerating drug approvals and market penetration, thereby significantly boosting the industry's growth
Ultra-Rare Pediatric Cancer Drug Market Analysis
- Ultra-rare pediatric cancer drugs, developed to treat highly specific and low-incidence malignancies in children, are increasingly vital in modern oncology due to their precision-targeted mechanisms, significant therapeutic potential, and alignment with advancements in genomics and personalized medicine
- The growing demand for these therapies is primarily fueled by rising awareness of pediatric rare cancers, increasing regulatory incentives such as orphan drug exclusivity and priority review vouchers, and greater pharmaceutical investment in high-impact, low-volume treatment pipelines
- North America dominated the ultra-rare pediatric cancer drug market with the largest revenue share of 41.7% in 2024, characterized by strong regulatory frameworks, early access to novel therapies, and a high concentration of specialized treatment centers, with the U.S. playing a central role in accelerating drug development through FDA-backed incentives and pediatric research networks
- Asia-Pacific is expected to be the fastest growing region in the ultra-rare pediatric cancer drug market during the forecast period due to increasing healthcare investments, expanding clinical trial infrastructure, and greater access to genetic testing and diagnostics in developing countries
- Leukemia segment dominated the ultra-rare pediatric cancer drug market with a market share of 40.1% in 2024, driven by the prevalence of subtypes such as acute lymphoblastic leukemia (ALL), availability of CAR-T therapies, and continued innovation in immunotherapy and gene-based treatments
Report Scope and Ultra-Rare Pediatric Cancer Drug Market Segmentation
|
Attributes |
Ultra-Rare Pediatric Cancer Drug Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Ultra-Rare Pediatric Cancer Drug Market Trends
“Breakthrough Therapies Through Genomic & Targeted Drug Advancements”
- A defining and accelerating trend in the global ultra-rare pediatric cancer drug market is the advancement of precision medicine and genomics-driven therapy development, enabling highly targeted treatments tailored to specific genetic mutations found in small pediatric cancer populations
- For instance, CAR-T cell therapies such as Kymriah (tisagenlecleucel) have shown transformative potential in treating relapsed acute lymphoblastic leukemia (ALL) in children, marking a major step in targeted immunotherapy for ultra-rare pediatric indications
- Increasing integration of next-generation sequencing (NGS) into pediatric oncology is enhancing early diagnosis and identifying actionable mutations. Pharmaceutical innovators are leveraging this data to create novel therapies that precisely address the molecular profiles of rare pediatric cancers, minimizing toxicity and maximizing outcomes
- The emergence of biotech startups and academic collaborations focused on pediatric oncology often supported by government grants and rare disease initiatives is fueling an expanding pipeline of gene therapies, tumor-specific monoclonal antibodies, and epigenetic modulators
- Organizations such as St. Jude Children’s Research Hospital and the Children’s Oncology Group are partnering with global pharmaceutical firms to accelerate pediatric clinical trials, drug repurposing, and biomarker discovery for ultra-rare cancers
- This paradigm shift toward personalized, genomics-informed, and biomarker-driven treatment strategies is reshaping drug development priorities and regulatory pathways in pediatric oncology. As a result, the demand for ultra-rare pediatric cancer drugs that demonstrate high specificity, fewer long-term side effects, and improved survival rates is rising steadily across developed healthcare systems
Ultra-Rare Pediatric Cancer Drug Market Dynamics
Driver
“Rising Research Funding and Regulatory Incentives Supporting Orphan Drug Development”
- The growing global attention to childhood cancers, particularly ultra-rare forms, is leading to an increase in funding, awareness initiatives, and public-private partnerships that aim to close the therapeutic gap for underserved pediatric populations
- For instance, the U.S. FDA's Rare Pediatric Disease Priority Review Voucher Program and Europe’s Orphan Medicinal Product Regulation are encouraging pharmaceutical companies to invest in niche oncology treatments by offering market exclusivity, fee waivers, and expedited approval processes
- These regulatory incentives are complemented by philanthropic funding, advocacy group lobbying, and institutional grants that support research in otherwise commercially unattractive segments. Initiatives such as Cancer Moonshot and partnerships with rare cancer foundations are directly facilitating early-stage research and clinical trial expansion
- Furthermore, the unmet medical need in this population, combined with growing public pressure for equitable access to life-saving therapies, is accelerating drug approvals, improving trial recruitment, and fostering innovation in pediatric formulations and delivery mechanisms
Restraint/Challenge
“Limited Patient Populations and High Development Costs”
- The inherently small patient base for ultra-rare pediatric cancers presents a significant challenge to commercial viability, often making it difficult for drug developers to recoup research and development investments
- In addition, clinical trial design and recruitment are constrained by the rarity and geographic dispersion of eligible patients, leading to longer development timelines and higher per-patient trial costs
- Regulatory requirements for safety and efficacy in pediatric populations further increase complexity, particularly in ensuring age-appropriate dosing, formulation, and ethical trial design
- While regulatory and philanthropic support is improving, the high cost of therapies such as gene or CAR-T treatments (often exceeding USD 1–2 million per patient) limits accessibility in lower-income regions and places pressure on reimbursement frameworks even in advanced healthcare systems
- Overcoming these challenges will require global collaboration, innovative clinical trial models adaptive regulatory approaches, and sustained investment from both public and private stakeholders to ensure that children with ultra-rare cancers receive timely and effective treatment options
Ultra-Rare Pediatric Cancer Drug Market Scope
The market is segmented on the basis of drug type, indication, treatment modality, and distribution channel.
- By Drug Type
On the basis of drug type, the ultra-rare pediatric cancer drug market is segmented into enzyme replacement therapies (ERT), gene therapies, monoclonal antibodies, small-molecule inhibitors, combination therapies, biologics, and non-biologics. The gene therapies segment held the largest revenue share in 2024, owing to its transformative role in treating genetic mutations associated with rare pediatric cancers. These therapies offer targeted and long-term solutions, particularly for hematologic malignancies such as relapsed acute lymphoblastic leukemia (ALL), where traditional therapies have limited efficacy. The introduction of CAR-T cell therapy, such as Kymriah, has accelerated market dominance in this category.
The monoclonal antibodies segment is projected to witness the fastest growth rate from 2025 to 2032, driven by increasing development of targeted immunotherapies for ultra-rare solid tumors and hematologic cancers. Their specificity in attacking cancer cells while sparing healthy tissues, coupled with improving biomarker-based diagnostics, is driving their clinical adoption across leading pediatric oncology centers.
- By Indication
On the basis of indication, the ultra-rare pediatric cancer drug market is categorized into leukemia, neuroblastoma, brain tumors, lymphoma, bone tumors, retinoblastoma, and others. The leukemia segment dominated the market in 2024, accounting for the largest revenue share of 40.1%. Acute lymphoblastic leukemia (ALL) is one of the most common pediatric cancers, and advancements in immunotherapies and gene-based treatments have significantly improved survival rates, especially in relapsed or refractory cases.
The brain tumor segment is anticipated to grow at the highest CAGR during the forecast period due to increased identification of rare central nervous system malignancies in children, rising precision-based approaches to treatment, and expanding clinical trial support for targeted therapies in this domain.
- By Treatment Modality
On the basis of treatment modality, the ultra-rare pediatric cancer drug market is segmented into chemotherapy, radiation therapy, immunotherapy, targeted therapy, and stem cell transplantation. The chemotherapy segment held the largest market share in 2024, as it remains the primary and widely accessible modality for most pediatric cancers. Despite growing precision medicine alternatives, chemotherapy is still the frontline therapy in many global treatment protocols due to its broad-spectrum effectiveness and cost efficiency.
The immunotherapy segment is projected to exhibit the fastest growth from 2025 to 2032, owing to the increasing success of therapies such as CAR-T and checkpoint inhibitors. These therapies offer enhanced survival with fewer long-term side effects and are rapidly being incorporated into front-line regimens for specific ultra-rare pediatric malignancies.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. The hospital pharmacies segment accounted for the largest revenue share in 2024, as most ultra-rare pediatric cancer drugs are administered in specialized oncology centers under medical supervision. These drugs often require strict handling protocols, physician oversight, and inpatient or infusion-based delivery models.
The online pharmacies segment is expected to grow at the fastest CAGR from 2025 to 2032, driven by the increasing digitalization of healthcare, improved access in remote regions, and growing support for at-home delivery of oral targeted therapies and supportive medications for pediatric oncology patients.
Ultra-Rare Pediatric Cancer Drug Market Regional Analysis
- North America dominated the ultra-rare pediatric cancer drug market with the largest revenue share of 41.7% in 2024, characterized by strong regulatory frameworks, early access to novel therapies, and a high concentration of specialized treatment centers
- The region benefits from high public and private investment in pediatric cancer research, widespread availability of genetic testing, and active participation in global clinical trials targeting rare and ultra-rare malignancies in children
- In addition, supportive policies such as the FDA's Rare Pediatric Disease Priority Review Voucher program and the presence of major pediatric oncology institutions have accelerated the approval and availability of novel therapies, making North America a central hub for innovation and access in the ultra-rare pediatric cancer drug market
U.S. Ultra-Rare Pediatric Cancer Drug Market Insight
The U.S. ultra-rare pediatric cancer drug market captured the largest revenue share of 81% in 2024 within North America, driven by a strong regulatory framework, advanced clinical research infrastructure, and significant public and private investment in rare disease therapies. The FDA’s Rare Pediatric Disease Priority Review Voucher program and robust support from institutions such as St. Jude and the Children's Oncology Group are accelerating the development and approval of breakthrough treatments. Growing access to genomic profiling and early diagnosis is also fueling demand for precision-targeted therapies among pediatric patients.
Europe Ultra-Rare Pediatric Cancer Drug Market Insight
The Europe ultra-rare pediatric cancer drug market is projected to expand at a substantial CAGR throughout the forecast period, supported by comprehensive orphan drug legislation, strong research collaborations, and increased awareness of pediatric rare cancers. The European Medicines Agency (EMA) continues to promote early-stage development through incentives and expedited regulatory pathways. The market is benefiting from rising investments in pediatric oncology research, especially in countries such as Germany, France, and the Netherlands, where specialized treatment centers and academic consortia are driving innovation.
U.K. Ultra-Rare Pediatric Cancer Drug Market Insight
The U.K. ultra-rare pediatric cancer drug market is anticipated to grow at a noteworthy CAGR during the forecast period, bolstered by a centralized healthcare system (NHS), strong rare disease strategies, and active engagement in pediatric clinical trials. The government’s Genomics England initiative and Rare Disease Framework are fostering early diagnosis and personalized treatment, encouraging the development and adoption of novel drugs targeting ultra-rare childhood cancers.
Germany Ultra-Rare Pediatric Cancer Drug Market Insight
The Germany ultra-rare pediatric cancer drug market is expected to expand at a considerable CAGR during the forecast period, driven by a well-established healthcare infrastructure, high awareness of pediatric oncology care, and consistent funding for research into rare malignancies. The country’s emphasis on precision medicine and sustainability in healthcare, coupled with strong collaborations between academic institutions and pharmaceutical companies, is advancing the development and accessibility of highly targeted therapies for ultra-rare pediatric cancer patients.
Asia-Pacific Ultra-Rare Pediatric Cancer Drug Market Insight
The Asia-Pacific ultra-rare pediatric cancer drug market is poised to grow at the fastest CAGR of 24% during the forecast period of 2025 to 2032, fueled by rising healthcare investments, rapid urbanization, and improved access to diagnostics and cancer care in key markets such as China, Japan, and India. Government-led digital health initiatives, expansion of pediatric oncology centers, and increased participation in global clinical trials are driving growth. In addition, local pharmaceutical manufacturers are beginning to invest in rare disease drug development, improving availability and affordability.
Japan Ultra-Rare Pediatric Cancer Drug Market Insight
The Japan ultra-rare pediatric cancer drug market is gaining momentum due to the country’s aging demographics, advanced healthcare infrastructure, and focus on innovative therapies. The government’s support for orphan drug development and integration of precision oncology into clinical practice are catalyzing growth. Japan’s regulatory alignment with global standards and strong biopharmaceutical R&D capacity are helping accelerate the approval of life-saving treatments for children with rare cancers.
India Ultra-Rare Pediatric Cancer Drug Market Insight
The India ultra-rare pediatric cancer drug market accounted for the largest market revenue share in Asia Pacific in 2024, attributed to a large pediatric population, rising awareness, and increasing government focus on rare diseases. Programs promoting early diagnosis, local manufacturing of oncology drugs, and participation in global research networks are expanding access to treatments. As India continues to develop pediatric oncology infrastructure and strengthen its clinical research ecosystem, the country is emerging as a key market for affordable and innovative solutions in ultra-rare childhood cancers.
Ultra-Rare Pediatric Cancer Drug Market Share
The ultra-rare pediatric cancer drug industry is primarily led by well-established companies, including:
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Bristol-Myers Squibb Company (U.S.)
- Sanofi (France)
- Amgen Inc. (U.S.)
- Johnson & Johnson Services, Inc. (U.S.)
- Lilly (U.S.)
- Takeda Pharmaceutical Company Limited (Japan)
- GSK plc (U.K.)
- Merck & Co., Inc. (U.S.)
- AbbVie Inc. (U.S.)
- Jazz Pharmaceuticals plc (Ireland)
- Blueprint Medicines Corporation (U.S.)
- Day One Biopharmaceuticals, Inc. (U.S.)
- PTC Therapeutics, Inc. (U.S.)
- Servier Laboratories (France)
- Y-mAbs Therapeutics, Inc. (U.S.)
- BeiGene, Ltd. (China)
- BridgeBio Pharma, Inc. (U.S.)
What are the Recent Developments in Global Ultra-Rare Pediatric Cancer Drug Market?
- In May 2024, Novartis AG announced positive results from its Phase II trial of a gene therapy candidate for treating relapsed pediatric neuroblastoma, marking a major milestone in advancing targeted treatments for ultra-rare childhood cancers. This development demonstrates the growing role of gene-based therapies in addressing critical gaps in pediatric oncology and showcases Novartis’s commitment to expanding its rare disease pipeline through precision medicine
- In April 2024, Pfizer Inc. entered into a strategic collaboration with Children’s Oncology Group to accelerate clinical trials for investigational drugs targeting ultra-rare pediatric malignancies such as Ewing sarcoma and hepatoblastoma. The partnership aims to reduce trial timelines and improve access to experimental therapies, reinforcing Pfizer’s dedication to addressing underserved patient populations in pediatric cancer care
- In March 2024, St. Jude Children’s Research Hospital launched the Global Pediatric Cancer Registry for Ultra-Rare Cancers, a groundbreaking initiative aimed at gathering clinical and genetic data on underrepresented malignancies. This registry enhances collaboration across international research institutions and is expected to accelerate biomarker discovery and the development of novel therapeutic approaches
- In February 2024, Day One Biopharmaceuticals received FDA Orphan Drug Designation for its lead candidate targeting pediatric high-grade glioma. This designation offers regulatory incentives, including tax credits and potential market exclusivity, and underscores the growing momentum behind innovative small-molecule and biologic treatments for ultra-rare childhood brain tumors
- In January 2024, Roche announced the expansion of its global pediatric cancer initiative, focusing on developing companion diagnostics for ultra-rare cancers. The initiative aims to improve early detection and patient stratification, enabling personalized therapies tailored to the genetic profile of each patient. This move reaffirms Roche’s leadership in precision oncology and its strategic focus on addressing critical unmet needs in pediatric oncology.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.