Global Urology Small Molecule Api Market
Market Size in USD Billion
CAGR :
% 
 USD
1.98 Billion
USD
2.86 Billion
2024
2032
USD
1.98 Billion
USD
2.86 Billion
2024
2032
| 2025 –2032 | |
| USD 1.98 Billion | |
| USD 2.86 Billion | |
|
|
|
|
Urology Small Molecule API Market Analysis
The Urology Small Molecule API market is primarily driven by the rising prevalence of urological disorders such as benign prostatic hyperplasia (BPH), prostate cancer, erectile dysfunction, and urinary tract infections (UTIs). The increasing global burden of these conditions, particularly among aging populations, is propelling the demand for effective small molecule therapies.
Advancements in drug discovery and the growing focus on targeted therapies are further fueling market growth. Small molecules offer high precision in targeting specific disease mechanisms, enhancing treatment efficacy with fewer side effects. As these drugs are generally more cost-effective than biologics, they are increasingly being adopted across healthcare systems, particularly in resource-limited settings.
The market is also witnessing a shift towards generics as patents for blockbuster drugs expire, offering more affordable treatment options. In addition, the rise of personalized medicine and innovations in drug formulations, such as extended-release tablets, present significant opportunities for growth.
However, challenges such as stringent regulatory approval processes, high R&D costs, and concerns over long-term safety may limit market expansion. Despite these hurdles, the urology small molecule API market remains poised for growth, driven by increasing disease prevalence and the need for effective, affordable treatments.
Urology Small Molecule API Market Size
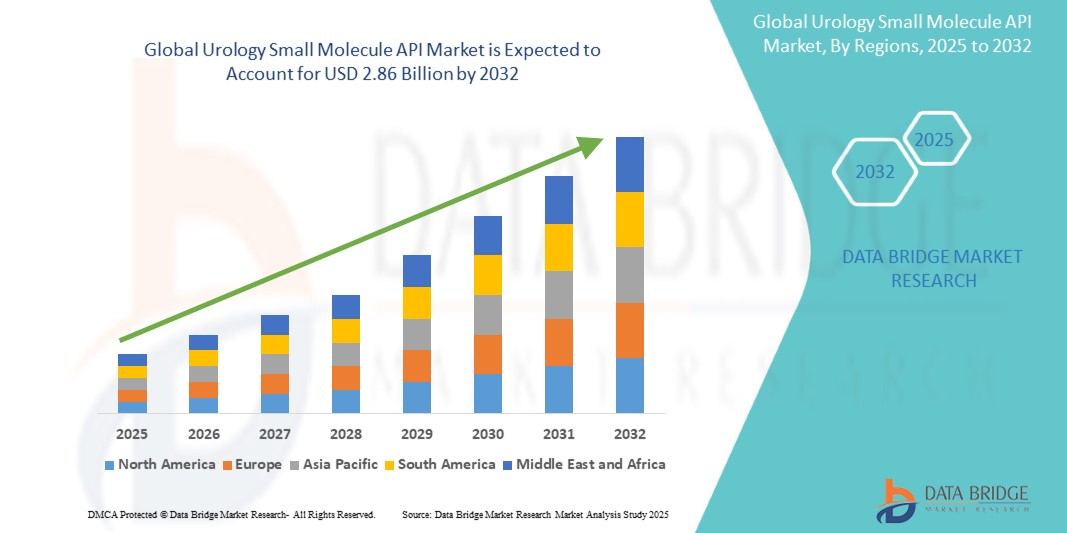
The global urology small molecule API market size was valued at USD 1.98 billion in 2024 and is projected to reach USD 2.86 billion by 2032, with a CAGR of 4.70% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Urology Small Molecule API Market Trends
“Increasing Prevalence of Urological Disorders”
The Urology Small Molecule API market is thriving due to the rising prevalence of urological disorders such as benign prostatic hyperplasia (BPH), prostate cancer, erectile dysfunction (ED), and urinary tract infections (UTIs). As aging populations grow, especially in developed regions, the demand for effective treatments for these conditions is increasing. Small molecules, celebrated for their cost-effectiveness, high efficacy, and ease of administration, offer a significant advantage over biologics, making them a preferred choice for both pharmaceutical companies and patients.
Recent advances in drug discovery and the rise of precision medicine are driving innovation in the development of targeted therapies, improving treatment outcomes while minimizing side effects. Furthermore, the growing adoption of generics, spurred by the patent expirations of major urology drugs, is increasing market accessibility. The expanding emphasis on affordable healthcare and the continuing clinical research into urological diseases further contributes to the ongoing growth of the market.
Report Scope and Urology Small Molecule API Market Segmentation
|
Attributes |
Urology Small Molecule API Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Italy, Spain, Denmark, Egypt, Finland, Netherlands, Nigeria, Poland, Sweden, Norway, Rest of Europe in Europe, China, Japan, India, South Korea, Australia, Thailand, Malaysia, Philippines, Vietnam, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Kuwait, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Key Market Players |
Accord Healthcare (UK), Alkem (India), AstraZeneca (UK), Aurobindo Pharma (India), Chartwell Pennsylvania, LP (U.S.), Dr. Reddy’s Laboratories Ltd. (India), Endo, Inc. (U.S.), GLENMARK PHARMACEUTICALS LTD. (India), Qilu Pharmaceutical Co., Ltd. (China), Johnson & Johnson Services, Inc. (U.S.), PT Actavis Indonesia (Indonesia), Sanofi (France), Sandoz Group AG (Switzerland), Sonnet BioTherapeutics, Inc. (U.S.), Sun Pharmaceutical Industries Ltd. (India), Synthon B.V. (The Netherlands), Teva Pharmaceutical Industries Ltd. (Israel), UroGen Pharma, Inc. (U.S.), Waylis Therapeutics (U.S.), Zydus Group (India) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Urology Small Molecule API Market Definition
Urology Small Molecule API (Active Pharmaceutical Ingredient) refers to the active pharmaceutical compounds used in the treatment of urological conditions, such as urinary tract infections, kidney stones, prostate issues, and bladder disorders. These small molecule APIs are typically chemically synthesized and work at the molecular level to interact with specific targets in the body to treat or manage urological diseases. Instances include drugs used for erectile dysfunction, benign prostatic hyperplasia, and overactive bladder. These small molecules are often administered in oral or injectable forms and are a key component in the development of pharmaceutical treatments in urology.
Urology Small Molecule API Market Dynamics
Drivers
- Growing Awareness and Early Diagnosis of Urological Conditions
Increasing awareness about urological conditions and the benefits of early diagnosis are driving the demand for Urology Small Molecule APIs. As more individuals become aware of symptoms such as frequent urination, erectile dysfunction, and pain during urination, there has been a rise in early diagnosis and treatment. This shift is leading to greater consumption of medications such as Tamsulosin for BPH and Sildenafil for erectile dysfunction. Public health campaigns and advancements in diagnostic tools are helping people detect urological issues early, allowing for better management of conditions. Greater awareness and early detection are accelerating the demand for effective treatments, driving the market for small molecule APIs used in urology.
- Advancements in Urology Drug Development
Technological advancements and ongoing research in urology have led to the development of new and more effective small molecule drugs. Research into more targeted therapies for conditions such as erectile dysfunction, BPH, and bladder cancer has resulted in innovations such as Sildenafil and Finasteride. These drugs have not only improved patient outcomes but have also opened doors for the development of more specialized treatments. Pharmaceutical companies are investing in R&D to develop drugs with fewer side effects and more precise mechanisms of action, such as PDE5 inhibitors for ED or alpha-blockers for BPH. Continuous advancements in urology drug development are enhancing treatment options and driving the market forward by attracting investments and expanding therapeutic areas.
Opportunities
- Expansion of Generic Urology Small Molecule Drugs
As patents for many urology drugs expire, the market for generic small molecule APIs presents significant opportunities. Generic alternatives to blockbuster drugs such as Tamsulosin (for BPH) and Sildenafil (for erectile dysfunction) can help reduce healthcare costs and make treatments more accessible to a broader patient base, especially in emerging markets. For instance, the availability of generic Sildenafil after the expiration of Viagra’s patent has made ED treatments more affordable globally. The increase in generic versions can lead to greater market penetration in developing regions, where cost-effective treatment options are essential. The growth of generic drugs is expected to drive market expansion by making urology treatments more affordable and accessible, especially in low- and middle-income countries.
- Rising Demand for Personalized Medicine
Personalized medicine is an emerging opportunity in the urology small molecule API market. With advancements in genomics and biotechnology, there is increasing interest in developing targeted therapies based on an individual’s genetic profile. For instance, drugs such as Finasteride, used for BPH, could be customized to suit specific genetic markers that affect a patient’s response to the medication. The rise in pharmacogenomics and personalized treatment options allows for better management of urological conditions, reducing adverse effects and improving outcomes. The move toward personalized medicine is expected to increase the demand for small molecule APIs, driving the development of more tailored therapies and improving patient satisfaction in the urology market.
Restraints/Challenges
- Stringent Regulatory Approval Processes
The approval process for urology small molecule APIs is highly regulated, with stringent requirements for clinical trials, safety, and efficacy. This can delay the launch of new drugs into the market and increase costs for pharmaceutical companies. For instance, the regulatory hurdles faced by new treatments for conditions such as prostate cancer or kidney stones can result in years of delay in drug availability. In addition, the need for extensive clinical data to prove the safety and effectiveness of new therapies often deters smaller companies from entering the market. These regulatory challenges slow down market growth, limit the introduction of innovative therapies, and increase the time to market for new small molecule drugs, affecting the overall expansion of the urology small molecule API market.
- High Cost of Research and Development
The high cost of research and development (R&D) for urology small molecule APIs presents a significant challenge. Developing new drugs for complex urological conditions, such as bladder cancer or erectile dysfunction, requires substantial investment in R&D, including extensive preclinical and clinical studies. For instance, developing small molecule drugs for rare urological diseases may require long timelines and significant funding, which can pose a financial risk for pharmaceutical companies. The high R&D costs create barriers for smaller companies to enter the market, and may also limit the number of new treatments available, potentially slowing market growth and limiting innovation in the urology sector.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Urology Small Molecule API Market Scope
The market is segmented on the basis of drug class, application, route of administration, manufacturing process, and end user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Drug Class
- Alpha-Blockers
- 5-Alpha Reductase Inhibitors
- Phosphodiesterase Type 5 Inhibitors
- Antibiotics
- Hormonal Therapies
- Others
Application
- Benign Prostatic Hyperplasia (BPH)
- Prostate Cancer
- Erectile Dysfunction
- Urinary Tract Infections
- Overactive Bladder
- Other Conditions
Route of Administration
- Oral
- Topical
- Injectable
Manufacturing Process
- Contract Manufacturing (CMO)
- In-house Manufacturing
End User
- Pharmaceutical Companies
- Contract Research Organizations (CROs)
- Hospitals and Clinics
- Other Healthcare Providers
Urology Small Molecule API Market Regional Analysis
The market is analysed and market size insights and trends are provided by drug class, application, route of administration, manufacturing process, and end user as referenced above.
The countries covered in the market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Italy, Spain, Denmark, Egypt, Finland, Netherlands, Nigeria, Poland, Sweden, Norway, Rest of Europe in Europe, China, Japan, India, South Korea, Australia, Thailand, Malaysia, Philippines, Vietnam, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Kuwait, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America is dominating the urology small molecule API market and is projected to maintain its position throughout the forecast period. This growth is driven by several factors, including the rising prevalence of kidney diseases, an increase in patient visits for both treatment and diagnosis, and the significant presence of major market players in the region. For instance, companies such as Boston Scientific and Medtronic continue to innovate and expand their product portfolios, further strengthening the market. The robust healthcare infrastructure in the U.S. also plays a crucial role in supporting this growth.
The Asia Pacific region is expected to exhibit the highest growth rate in urology small molecule API market due to several factors, including greater awareness of kidney and urinary diseases, higher healthcare spending, and improved medical infrastructure. The rising prevalence of conditions such as nerve damage and urinary tract infections (UTIs) is also likely to drive market growth. UTIs, in particular, are among the most common infections in this region, further contributing to the market's expansion.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Urology Small Molecule API Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Urology Small Molecule API Market Leaders Operating in the Market Are:
- Accord Healthcare (UK)
- Alkem (India)
- AstraZeneca (UK)
- Aurobindo Pharma (India)
- Chartwell Pennsylvania, LP (U.S.)
- Dr. Reddy’s Laboratories Ltd. (India)
- Endo, Inc. (U.S.)
- GLENMARK PHARMACEUTICALS LTD. (India)
- Qilu Pharmaceutical Co., Ltd. (China)
- Johnson & Johnson Services, Inc. (U.S.)
- PT Actavis Indonesia (Indonesia)
- Sanofi (France)
- Sandoz Group AG (Switzerland)
- Sonnet BioTherapeutics, Inc. (U.S.)
- Sun Pharmaceutical Industries Ltd. (India)
- Synthon B.V. (The Netherlands)
- Teva Pharmaceutical Industries Ltd. (Israel)
- UroGen Pharma, Inc. (U.S.)
- Waylis Therapeutics (U.S.)
- Zydus Group (India)
Latest Developments in Urology Small Molecule API Market
- In December 2024, UroGen Pharma Ltd. announced the presentation of the Phase 3 ENVISION trial’s efficacy and safety results at the Society of Urologic Oncology (SUO) annual meeting in Dallas, TX. In the study, UGN-102 treatment showed an 82.3% (95% CI: 75.9%, 87.1%) duration of response (DOR) at 12 months, according to the Kaplan-Meier estimate, in patients who achieved a complete response (CR) at 3 months following the initial treatment with UGN-102.
- In Oct 2024, Zydus Lifesciences announced that, it has received approval from the US health regulator to produce a generic prostate cancer treatment drug. The company has received tentative approval from the US Food and Drug Administration (USFDA) to manufacture Enzalutamide tablets (40 mg and 80 mg), the drug maker said in a regulatory filing.
- In Oct 2024, Aurobindo Pharma announced that it has received final approval from the US Food & Drug Administration (USFDA) to manufacture and market Cephalexin Tablets USP, 250 mg. The approved drug is bioequivalent and therapeutically equivalent to the reference listed drug (RLD), Keflet Tablets, 250 mg and 500 mg, of Eli Lilly and company. The product will be launched in Q3FY25.
- In June 2024, Palatin Technologies, Inc. announced the launch of a Phase 2 clinical study for bremelanotide (BMT), a melanocortin 4 receptor (MC4R) agonist, when co-administered with a phosphodiesterase 5 inhibitor (PDE5i) to treat erectile dysfunction (ED) in patients who do not respond to PDE5i monotherapy. Topline results from the Phase 2 study are anticipated by the end of 2024.
- In April 2024, the U.S. Food and Drug Administration approved Pivya (pivmecillinam) tablets for treating uncomplicated urinary tract infections (UTIs) in adult females, caused by susceptible strains of Escherichia coli, Proteus mirabilis, and Staphylococcus saprophyticus. Uncomplicated UTIs are bacterial infections of the bladder in women without any structural abnormalities in the urinary tract. The FDA approval of Pivya was granted to UTILITY Therapeutics Ltd.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.