Global Ustekinumab Market
Market Size in USD Billion
CAGR :
% 
 USD
12.07 Billion
USD
19.09 Billion
2024
2032
USD
12.07 Billion
USD
19.09 Billion
2024
2032
| 2025 –2032 | |
| USD 12.07 Billion | |
| USD 19.09 Billion | |
|
|
|
|
Ustekinumab Market Size
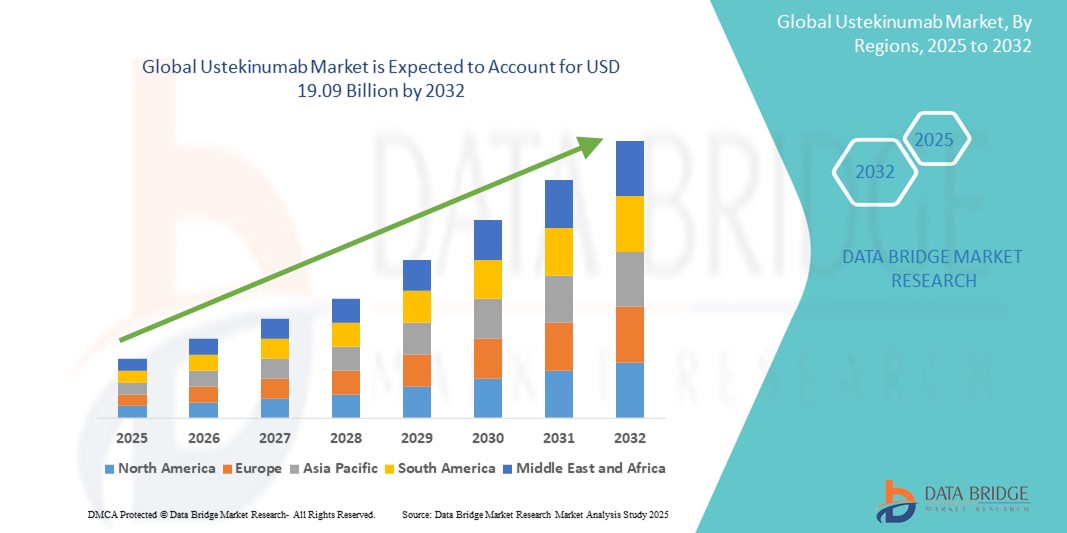
- The global ustekinumab market size was valued at USD 12.07 billion in 2024 and is expected to reach USD 19.09 billion by 2032, at a CAGR of 5.90% during the forecast period
- The market growth is largely fueled by the growing adoption and technological progress within biologics and targeted immunotherapies, leading to increased precision and efficacy in treating chronic autoimmune conditions such as psoriasis, Crohn’s disease, and ulcerative colitis
- Furthermore, rising patient demand for long-term, low-frequency dosing treatments that provide sustained symptom relief is establishing ustekinumab as the preferred monoclonal antibody therapy across key therapeutic areas. These converging factors are accelerating the uptake of ustekinumab solutions, thereby significantly boosting the industry's growth
Ustekinumab Market Analysis
- Ustekinumab, a monoclonal antibody targeting interleukin-12 and interleukin-23, has become a vital component in the treatment of immune-mediated inflammatory diseases such as plaque psoriasis, psoriatic arthritis, Crohn’s disease, and ulcerative colitis. Its targeted mechanism of action offers patients long-term remission, reduced flare-ups, and improved quality of life, establishing it as a preferred biologic therapy in both hospital and specialty care settings
- The escalating demand for Ustekinumab is primarily driven by the growing prevalence of chronic autoimmune disorders, increasing awareness among patients and physicians, and the expanding accessibility of advanced biologic therapies across emerging markets. In addition, the shift toward personalized medicine and real-world evidence supporting Ustekinumab’s efficacy and safety further strengthens its adoption
- North America dominated the Ustekinumab market with the largest revenue share of 22.4% in 2024, attributed to the high incidence of inflammatory bowel disease and psoriasis, robust reimbursement frameworks, and early adoption of biologic therapies. The United States, in particular, leads the region due to strong clinical infrastructure, high treatment awareness, and a concentration of key biopharmaceutical players actively promoting long-term therapy adherence
- Asia-Pacific is expected to be the fastest-growing region in the ustekinumab market during the forecast period, owing to increasing urbanization, rising disposable incomes, greater healthcare access, and growing diagnosis rates for autoimmune diseases. Countries such as China, Japan, and India are witnessing significant investments in biologic drug infrastructure and regulatory acceleration, fostering market expansion
- The Adult segment dominated the ustekinumab market, with the market share of 85.1% in 2024, driven by the high incidence of psoriasis and Crohn’s disease among adults and established clinical evidence supporting ustekinumab’s long-term safety and efficacy in this population
Report Scope and Ustekinumab Market Segmentation
|
Attributes |
Ustekinumab Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Ustekinumab Market Trends
“Growing Preference for Targeted Biologic Therapies”
- A significant and accelerating trend in the global ustekinumab market is the increasing adoption of targeted biologic therapies that provide long-term control over chronic autoimmune and inflammatory diseases such as Crohn’s disease, ulcerative colitis, and plaque psoriasis. Ustekinumab, a human monoclonal antibody targeting interleukin-12 and interleukin-23, has become a preferred treatment due to its durable efficacy, favorable safety profile, and less frequent dosing regimen
- For instance, Ustekinumab is the only biologic currently approved for both Crohn’s disease and ulcerative colitis that targets the IL-12/23 pathway, providing consistent therapeutic benefits across multiple inflammatory conditions. Its dual cytokine inhibition has proven effective in patients who have failed other lines of therapy, making it an essential option in treatment-resistant cases
- The increasing use of real-world evidence and long-term extension studies is reinforcing physician confidence in Ustekinumab, especially for maintaining remission and improving patient quality of life. Clinical programs continue to highlight its benefits in reducing steroid dependency, preventing disease progression, and supporting mucosal healing in gastrointestinal conditions
- Healthcare systems globally are beginning to incorporate biologic optimization strategies, wherein patient biomarkers and disease activity levels guide the initiation and continuation of Ustekinumab therapy. This personalized treatment approach is helping providers improve therapeutic outcomes while minimizing unnecessary drug exposure
- The integration of Ustekinumab into updated clinical treatment guidelines by leading associations such as the American Gastroenterological Association (AGA), European Crohn’s and Colitis Organisation (ECCO), and National Psoriasis Foundation (NPF) underscores its importance in standard of care. Its role is also expanding in pediatric populations and off-label use, supported by emerging clinical data
- The demand for advanced biologics such as Ustekinumab is expected to continue rising, particularly across emerging markets, as biosimilar competition remains limited and patient access improves through expanded insurance coverage and government reimbursement programs
Ustekinumab Market Dynamics
Driver
“Growing Need Due to Rising Prevalence of Chronic Inflammatory Diseases”
- The increasing global burden of autoimmune and inflammatory disorders—such as Crohn’s disease, ulcerative colitis, plaque psoriasis, and psoriatic arthritis—is a major driver fueling the demand for Ustekinumab. As more patients seek effective long-term disease management, biologic therapies such as Ustekinumab are becoming critical components of chronic care strategies
- For instance, in April 2024, Janssen Biotech Inc. (Johnson & Johnson) expanded Ustekinumab’s indication to include pediatric patients with active psoriatic arthritis in several global markets. This regulatory milestone reflects the growing emphasis on early intervention in autoimmune conditions and further expands the drug’s target patient pool, thereby contributing to global market growth
- With patients and healthcare professionals prioritizing therapies that offer durable remission, minimal side effects, and infrequent dosing schedules, Ustekinumab stands out due to its IL-12/23 inhibition mechanism and proven efficacy across multiple indications. Its favorable benefit-risk profile makes it a first-line or second-line option in both gastroenterology and dermatology practices
- In addition, growing treatment awareness, increased access to specialist care, and the expansion of specialty pharmacy services are contributing to higher adoption rates in both developed and emerging markets. Real-world data supporting treatment persistence and improved quality of life further enhances Ustekinumab’s market appeal
- The convenience of subcutaneous administration at home, combined with patient support programs and biologic optimization strategies, encourages long-term adherence and treatment satisfaction. As healthcare providers and payers shift toward value-based care models, the clinical and economic effectiveness of Ustekinumab positions it favorably within treatment guidelines and reimbursement policies
Restraint/Challenge
“High Cost and Competitive Biologic Landscape”
- One of the key challenges to broader market penetration for ustekinumab is its high cost, especially in regions with limited reimbursement infrastructure or out-of-pocket payment systems. Biologic therapies, though effective, often face resistance in cost-sensitive markets due to their premium pricing
- For instance, in some low- and middle-income countries, limited public funding for advanced biologics results in slower adoption, with physicians opting for older, less costly treatments unless biosimilars or patient access programs are available. This creates disparities in treatment availability across regions
- In addition, Ustekinumab faces rising competition from other biologics and newer targeted therapies such as IL-23-only inhibitors and JAK inhibitors, which are gaining traction for specific indications. As the therapeutic landscape evolves, maintaining a competitive edge will require continued evidence generation, label expansion, and pricing strategies
- Another ongoing challenge is the delay in access caused by complex prior authorization and specialty pharmacy distribution channels, which can impact patient initiation and long-term adherence
- Overcoming these barriers will require expanded reimbursement coverage, biosimilar development, cost-effectiveness data, and increased investment in patient education and physician engagement
Ustekinumab Market Scope
The market is segmented on the basis of drug class, demographic, application, dosage form, end-users, and distribution channel.
• By Drug Class
On the basis of drug class, the Ustekinumab market is segmented into interleukin inhibitors and others. The interleukin inhibitors segment dominated the market with a 72.8% revenue share in 2024, owing to the high efficacy of ustekinumab in targeting IL-12 and IL-23 pathways in autoimmune disorders.
The Others segment is projected to grow at a CAGR of 4.6% from 2025 to 2032, supported by novel biologics entering the market.
• By Demographic
On the basis of demographic, the Ustekinumab market is segmented into adult and pediatric. The adult segment held the largest share at 85.1% in 2024, driven by the high incidence of psoriasis and Crohn’s disease among adults.
The pediatric segment is anticipated to grow at a fastest CAGR of 7.3% from 2025 to 2032, supported by regulatory expansions and rising diagnosis rates in children.
• By Application
On the basis of application, the market is segmented into arthritis, colitis, lupus erythematosus, myositis multiplex with palmoplantar pustulosis, cirrhosis of the liver, sarcoidosis, diabetes, and others. The Colitis segment (including Crohn’s disease and ulcerative colitis) dominated with a 39.5% share in 2024, due to increasing global prevalence and clinical success of ustekinumab.
The Arthritis segment is anticipated to grow at a fastest CAGR of from 2025 to 2032, supported by regulatory expansions and rising diagnosis rates in children
• By Dosage Form
On the basis of dosage form, the market is segmented into intravenous solution and subcutaneous solution. The Subcutaneous Solution segment led the market with a 64.2% revenue share in 2024, driven by greater patient convenience, ease of self-administration, and reduced dependence on hospital visits.
The intravenous solution segment is projected to grow at a fastest CAGR of 4.1% from 2025 to 2032, primarily utilized for induction therapy and acute management and its efficacy in critical care and complex conditions.
• By End Users
On the basis of end users, the market is segmented into hospitals, clinics, and others. The Hospitals segment held the largest revenue share at 58.7% in 2024, due to their role in initiating biologic therapies and performing intravenous administrations.
The Clinics segment is expected to grow at a fastest CAGR of 6.4% from 2025 to 2032, aligned with increasing adoption of subcutaneous therapies and outpatient treatments.
• By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The Hospital Pharmacy segment dominated with a 51.6% revenue share in 2024, due to institutional purchasing and on-site dispensing.
The Online Pharmacy segment is anticipated to expand at the fastest CAGR of 8.2% from 2025 to 2032, owing to improved access and home delivery convenience.
Ustekinumab Market Regional Analysis
- North America dominated the Ustekinumab market with a revenue share of 22.4% in 2024, driven by robust demand for biologic therapies and established healthcare infrastructure
- The U.S. alone captured 83.1% of North America’s market in 2024, fueled by high healthcare adoption, favorable reimbursement policies, and growing patient demand for long-term autoimmune disease management
- Greater physician awareness, extensive biologics access, and supportive health insurance frameworks further bolstered Ustekinumab's prominence in the region
U.S. Ustekinumab Market Insight
The U.S. ustekinumab market accounted for 83.1% market share in the North American Market in 2024. Growth in the U.S. is propelled by the widespread use of specialty biologics, early FDA approvals, robust insurance coverage, and an increasing number of patients diagnosed with immune-mediated diseases. The country also benefits from Janssen’s strong distribution network, patient support programs, and continuous investment in R&D, clinical trials, and label expansions.
Europe Ustekinumab Market Insight
The Europe ustekinumab market held a market share of 27.3% in 2024, positioning it as the second-largest regional contributor. Strong clinical adoption, regulatory alignment under the EMA, and robust physician training across Western Europe have supported Ustekinumab’s continued growth. Hospital formularies increasingly include Ustekinumab in first-line biologic options for moderate to severe IBD and psoriasis cases.
U.K. Ustekinumab Market Insight
The U.K. ustekinumab market contributed 3.3% to the global market in 2024, with growing adoption in NHS-run inflammatory disease programs. Rising patient preference for home-administered subcutaneous biologics and streamlined NHS pathways are accelerating market penetration.
Germany Ustekinumab Market Insight
The Germany ustekinumab market accounted for 4.1% of the global market share in 2024. Strong healthcare expenditure, specialist access, and biosimilar interest are key factors supporting adoption, especially in dermatology clinics and gastroenterology centers.
Asia-Pacific Ustekinumab Market Insight
The Asia-Pacific ustekinumab market was the fastest-growing region, in 2024 and a projected CAGR of 5.9% from 2025 to 2032. The region is experiencing significant momentum due to rising healthcare investment, increasing diagnosis rates, and local biologic production. Initiatives across China, India, Japan, and South Korea to expand access to advanced therapies are playing a crucial role.
Japan Ustekinumab Market Insight
The Japan ustekinumab market held a global market share of 6.3% in 2024, driven by high disease burden, strong hospital infrastructure, and early biologic adoption. The Ministry of Health’s support for immunomodulators and continued expansion of specialty pharmacy access fuels uptake.
China Ustekinumab Market Insight
The China ustekinumab market captured the largest share within Asia-Pacific, at approximately 10% of the global market in 2024. Factors such as increasing disposable income, rising prevalence of autoimmune diseases, and rapid expansion of domestic biologics production are boosting growth. Government initiatives around "Healthy China 2030" and insurance expansion further promote treatment accessibility.
Ustekinumab Market Share
The Ustekinumab industry is primarily led by well-established companies, including:
- Fuji Pharma Co., Ltd. (Japan)
- Meiji Holdings Co., Ltd. (Japan)
- Mitsubishi Tanabe Pharma Corporation (Japan)
- STADA Arzneimittel AG (Germany)
- Formycon AG (Germany)
- NeuClone (Australia)
- Outlook Therapeutics, Inc. (U.S.)
- BioXpress Therapeutics SA (Switzerland)
- Johnson & Johnson Private Limited (U.S.)
Latest Developments in Global Ustekinumab Market
- In July 2025, Teva Pharmaceuticals and Alvotech announced that the U.S. FDA granted interchangeability status for SELARSDI (ustekinumab-aekn) with Stelara (ustekinumab). This approval allows pharmacy-level substitution without prescriber intervention, marking a major step forward in biosimilar acceptance and increasing access to cost-effective treatment options for autoimmune conditions such as psoriasis and Crohn’s disease
- In July 2025, Johnson & Johnson submitted a U.S. FDA application seeking approval of Stelara (ustekinumab) for the treatment of pediatric Crohn’s disease. This move aims to expand the drug’s therapeutic indications and offer a vital treatment alternative for younger patients with inflammatory bowel disease
- In July 2025, Biocon Biologics and Yoshindo announced a collaboration to expand access to a ustekinumab biosimilar in Japan. This partnership aims to address the rising demand for cost-effective biologic treatments and support broader healthcare affordability in Japan’s chronic inflammatory disease segment
- In July 2025, Bio-Thera Solutions and Hikma Pharmaceuticals received U.S. FDA approval for Starjemza (ustekinumab-hmny), a biosimilar referencing Stelara. This approval signifies a major milestone for both companies, allowing broader access to biosimilar therapies in the U.S. market and fostering competition
- In July 2025, Celltrion announced FDA approval of an additional presentation of Steqeyma (ustekinumab-stba), specifically tailored for pediatric dosing flexibility. This new presentation enhances treatment personalization for younger patients with autoimmune conditions, expanding access to Celltrion’s biosimilar therapy in the U.S.
- In July 2025, a Pharmaceutical Technology report covered the FDA approval of SELARSDI, noting its interchangeable designation with Stelara as a landmark event in the biosimilars industry. The article highlighted the significance of this approval in enhancing affordability and streamlining access to critical biologics for U.S. patients
- In July 2024, Celltrion announced that Health Canada granted approval for its new drug submission (NDS) for Steqeyma (development name CT-P43), a biosimilar designed for treating autoimmune diseases. This biosimilar is modeled after Stelara, expanding treatment options for patients with conditions such as psoriasis and Crohn's disease. The approval represents a significant milestone for Celltrion, enhancing access to effective therapies for managing autoimmune disorders in Canada
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.