Uveitis Drug Market Size
-
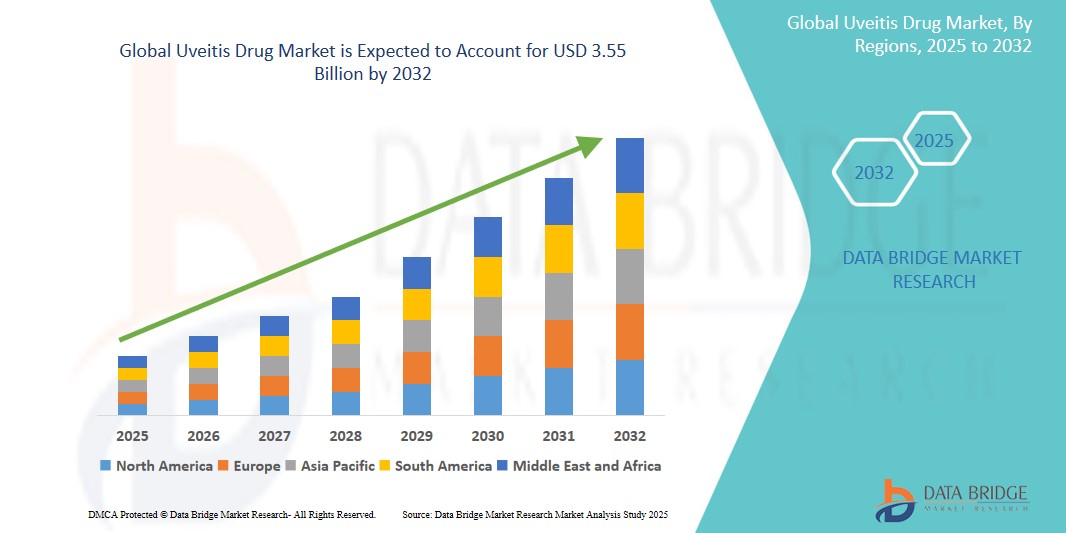
The global Uveitis Drug Market was valued at USD 1.87 billion in 2024 and is expected to reach USD 3.55 billion by 2032.
- During the forecast period of 2025 to 2032, the market is projected to grow at a CAGR of 8.2%, primarily driven by increasing incidence of autoimmune disorders, enhanced diagnostic capabilities, and the growing adoption of targeted biologic therapies.
- This growth is fueled by factors such as advances in immunology, rising awareness of ocular health, and increasing aging population prone to chronic inflammatory eye conditions.
Uveitis Drug Market Analysis
- The global Uveitis Drug Market is anticipated to gain significant momentum during the forecast period, with a CAGR of 8.2% from 2025 to 2032.
- Uveitis—comprising anterior, intermediate, posterior, and panuveitis types—is a major cause of preventable vision loss, often associated with systemic autoimmune diseases such as sarcoidosis, ankylosing spondylitis, and Behçet’s disease.
- The market is being supported by increasing demand for novel corticosteroid-sparing therapies, expanding pipelines of immunomodulatory drugs, and development of sustained-release formulations.
- Increasing healthcare expenditure, improved access to ophthalmologic care, and expanding reimbursement policies in emerging economies are expected to further accelerate market growth
Report Scope and Gastrointestinal Small Molecule API Market Segmentation
|
Attributes |
Medication-Assisted Treatment (MAT) Key Market Insights |
|
Segments Covered |
• By Drug: Corticosteroids, Immunosuppressants, Biologics, Cycloplegic Agents, Others |
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include import export analysis, production capacity overview, production consumption analysis, price trend analysis, climate change scenario, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Uveitis Drug Market Trends
“Shift Toward Biologic and Targeted Immunomodulatory Therapies”
-
A prominent trend in the Uveitis Drug Market is the increasing adoption of biologic agents and targeted therapies, particularly in non-infectious uveitis cases that are refractory to conventional corticosteroids.Treatments now increasingly focus on TNF-alpha inhibitors, IL-6 antagonists, and T-cell modulators to address the underlying immune pathways with greater specificity.
- For instance, adalimumab, a TNF-alpha inhibitor, has been FDA-approved for non-infectious intermediate, posterior, and panuveitis, offering sustained remission and reduced flare-ups.
- The integration of OCT imaging, AI-based diagnostics, and personalized dosing algorithms is enhancing the precision of disease monitoring and treatment adjustment.
- Additionally, the development of depot injections and ocular implants delivering sustained drug release is improving patient compliance and reducing systemic side effects.
Uveitis Drug Market Dynamics
Driver
“Growing Prevalence of Autoimmune and Inflammatory Eye Diseases”
- The global burden of inflammatory eye conditions is increasing due to a rise in autoimmune diseases and infectious triggers that affect ocular tissues.
- For instance, According to the American Academy of Ophthalmology (2023), uveitis accounts for 10–15% of blindness in the developed world, with autoimmune disorders such as lupus and rheumatoid arthritis being major contributors.
- The chronic and recurrent nature of uveitis necessitates long-term management strategies, propelling the demand for reliable, fast-acting, and steroid-sparing therapies.
- “Regulatory Advancements and Fast-Track Designations for Biologics”
- Supportive regulatory pathways and increasing investment in orphan drug development are expediting the approval of innovative treatments for rare uveitic conditions.
- For example: In 2022, the FDA granted orphan drug designation and fast-track status to multiple novel biologics for the treatment of non-infectious posterior uveitis, reinforcing the push toward targeted treatment modalities and improved patient access
Gastrointestinal Small Molecule API Market Scope
The market is segmented on the basis of type, drug class, , route of administration, end-users, and distribution channel.
|
Segmentation |
Sub-Segmentation |
|
Drug Class |
|
|
Route of Administration |
|
|
End-Users |
|
|
Distribution Channel |
|
Global Uveitis Drug Market Regional Analysis
“North America is the Dominant Region in the Uveitis Drug Market”
- North America dominates the global Uveitis Drug market owing to its advanced pharmaceutical ecosystem, robust healthcare infrastructure, and high awareness of ocular health and uveitis management.
- The United States holds the largest market share in this region, driven by a high incidence of uveitis, availability of specialized ophthalmologists, and rising cases of autoimmune conditions linked with uveitis.
- The region benefits from substantial R&D funding, presence of major drug manufacturers, and a strong regulatory environment led by the U.S. Food and Drug Administration (FDA), which ensures drug safety and efficacy.
- Increasing demand for biologics, corticosteroids, and immunosuppressive therapies along with rising adoption of advanced diagnostic tools contributes to continued market expansion.
- Collaborations between academic research institutions and pharmaceutical giants, alongside clinical advancements and innovation in drug delivery systems, are fostering growth in this region.
“Asia-Pacific is Projected to Register the Highest Growth Rate”
The Asia-Pacific region is expected to exhibit the fastest growth in the Uveitis Drug market, owing to increasing healthcare investments, a growing patient population, and improving access to specialty care.
- Key countries such as India, China, Japan, and South Korea are leading this growth, supported by rising awareness of uveitis, increasing prevalence of infectious and non-infectious forms of the disease, and improved ophthalmology services.
- Supportive government policies to enhance domestic pharmaceutical production and reduce reliance on imports are accelerating regional development.
- Japan, with its aging population and high healthcare standards, has shown strong adoption of advanced uveitis treatments and contributes significantly to regional market progress.
- A surge in clinical trials, expansion of biosimilar manufacturing, and collaborations between global pharmaceutical leaders and local players are expected to drive long-term growth across Asia-Pacific.
Uveitis Drug Market Share
The competitive landscape provides comprehensive insights into key market players, detailing their profiles, financial data, R&D efforts, product portfolios, operational footprint, manufacturing capacity, strategic initiatives, strengths, weaknesses, and contributions to the uveitis treatment segment.
The Major Market Leaders Operating in the Market Include:
- AbbVie Inc.
- Novartis AG
- Bausch + Lomb
- Johnson & Johnson Services, Inc.
- Allergan (a subsidiary of AbbVie)
- Santen Pharmaceutical Co., Ltd.
- Eyegate Pharmaceuticals, Inc.
- EyePoint Pharmaceuticals, Inc.
- Alimera Sciences
- Clearside Biomedical, Inc.
- Teva Pharmaceutical Industries Ltd.
- Sun Pharmaceutical Industries Ltd.
- Pfizer Inc.
- Roche Holding AG
- Regeneron Pharmaceuticals, Inc.
- Amgen Inc.
- Acelyrin, Inc.
- MeiraGTx Holdings plc
- Ocugen, Inc.
- Aldeyra Therapeutics, Inc.
Latest Developments in Global Uveitis Drug Market
- In March 2022, a report from the International Agency for the Prevention of Blindness outlined significant challenges in the uveitis drug sector, including gaps in early diagnosis, high treatment costs, and the complexity of managing chronic and recurrent forms of the disease.
The report highlighted the growing importance of targeted biologics, intravitreal delivery systems, and personalized medicine in reducing relapses and improving long-term outcomes. Industry focus is increasingly shifting toward non-steroidal alternatives and disease-modifying therapies to minimize adverse effects and enhance patient quality of life.
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.