Global Vaginitis Therapeutics Market
Market Size in USD Billion
CAGR :
% 
 2.60 USD
4.75 USD
2024
2032
2.60 USD
4.75 USD
2024
2032
| 2025 –2032 | |
| 2.60 USD | |
| 4.75 USD | |
|
|
|
|
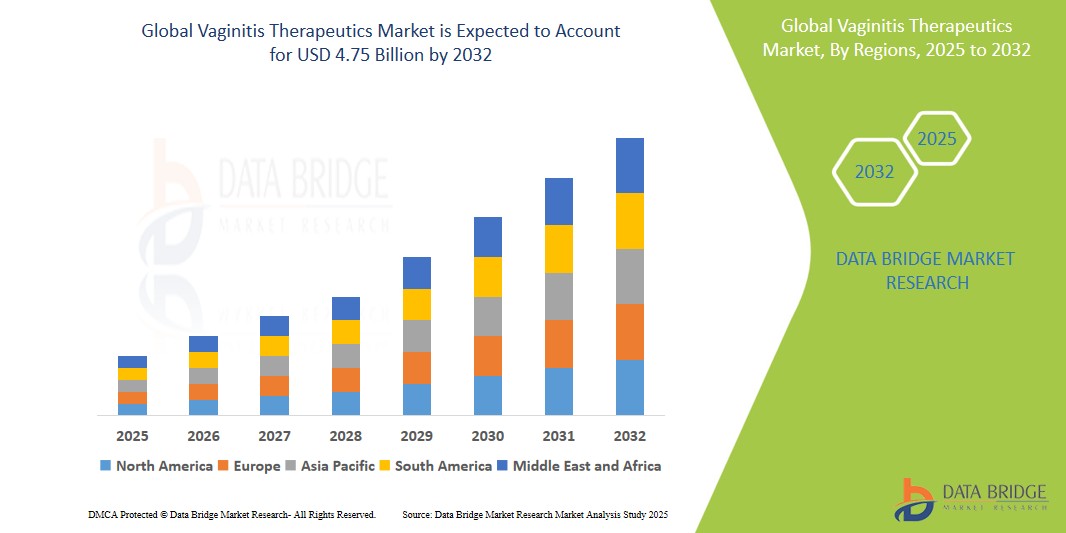
Vaginitis Therapeutics Market Size
- The global Vaginitis Therapeutics Market size was valued at USD 2.60 billion in 2024 and is expected to reach USD 4.75 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 7.8% during the forecast period
- Market growth is largely driven by the increasing global prevalence of various types of vaginitis, including bacterial vaginosis, vulvovaginal candidiasis, and trichomoniasis.
- Furthermore, rising awareness about women's reproductive health, improved diagnostic techniques, and the availability of diverse therapeutic options are establishing effective vaginitis management as a key focus area in gynecological care. These converging factors are accelerating the demand for and uptake of vaginitis therapeutics, thereby significantly boosting the industry's growth
Vaginitis Therapeutics Market Analysis
- Vaginitis is an inflammation of the vagina that can result in discharge, itching, and pain. It is most commonly caused by bacterial vaginosis, yeast infections, or trichomoniasis, though it can also be due to non-infectious causes
- Treatment involves addressing the underlying cause with specific medications. The escalating demand for Vaginitis Therapeutics is primarily fueled by improved diagnostic tools leading to earlier identification, increased patient awareness, and a robust pipeline of novel drug formulations offering better efficacy and fewer side effects.
- North America dominates the Vaginitis Therapeutics Market with the largest revenue share of 40.01% in 2025, characterized by a high prevalence of vaginitis, advanced healthcare infrastructure, and strong patient awareness and access to gynecological care
- The U.S. is experiencing substantial growth in Vaginitis Therapeutics, particularly in gynecology clinics and primary care settings, driven by widespread availability of prescription and over-the-counter treatments and robust pharmaceutical innovation
- Asia-Pacific is expected to be the fastest-growing region in the Vaginitis Therapeutics Market during the forecast period due to increasing awareness of women's health issues, improving access to healthcare, and a large patient pool in countries like China and India
- The Antibiotics segment is expected to dominate the Vaginitis Therapeutics Market with a market share of 43.2% in 2025, driven by the high prevalence of bacterial vaginosis and trichomoniasis, both of which primarily require antibiotic treatment.
- Report Scope and Vaginitis Therapeutics Market Segmentation
|
Attributes |
Vaginitis Therapeutics Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Vaginitis Therapeutics Market Trends
“Rise in Self-Care and Over-the-Counter (OTC) Solutions”
- A significant and accelerating trend in the global Vaginitis Therapeutics Market is the increasing preference for self-care and the growing availability and adoption of Over-the-Counter (OTC) solutions for common types of vaginitis, particularly vulvovaginal candidiasis (yeast infections)
- This shift is driven by greater patient awareness, desire for convenience, and the de-stigmatization of women's health issues
- For instance, in April 2024, a leading consumer health report indicated a substantial rise in OTC antifungal sales for vaginal yeast infections, driven by direct-to-consumer marketing and online accessibility. Such widespread adoption is expected to propel the Vaginitis Therapeutics industry growth in the forecast period. OTC solutions offer features such as immediate access, discretion, and cost-effectiveness, providing a compelling upgrade over the need for a doctor's visit for initial diagnosis and prescription
- the growing popularity of digital health platforms offering symptom checkers and educational resources is making self-care an integral component of women's health management, offering seamless integration with pharmacist consultations and telemedicine follow-ups when necessary. The convenience of various formulations (creams, suppositories, oral tablets), the empowering nature of self-treatment, and the increasing availability of clear usage instructions are key factors propelling the adoption of OTC products
- The trend towards personalized health management and the increasing availability of scientifically backed OTC remedies further contribute to market growth.
Vaginitis Therapeutics Market Dynamics
Driver:
“Increasing Prevalence of Vaginal Infections Globally”
- The increasing global prevalence of various types of vaginal infections, particularly bacterial vaginosis, vulvovaginal candidiasis, and trichomoniasis, is a significant driver for the heightened demand for Vaginitis Therapeutics
- For instance, in April 2024, the World Health Organization (WHO) released updated statistics highlighting the significant global burden of sexually transmitted infections (STIs) and other vaginal infections, underscoring the widespread need for effective treatments. Such reports by key international health organizations are expected to drive the Vaginitis Therapeutics industry growth in the forecast period
- As healthcare providers and patients become more aware of the commonality and potential complications of untreated vaginitis, advanced therapeutic solutions offer features such as targeted antimicrobial action, symptom relief, and prevention of recurrence, providing a compelling upgrade over older, less effective remedies
- Furthermore, the growing emphasis on women's reproductive health and the desire for improved quality of life are making Vaginitis Therapeutics an integral component of primary and gynecological care, offering seamless integration with routine check-ups and sexual health counseling
- The convenience of diverse drug formulations, the availability of both prescription and OTC options, and the increasing accessibility of diagnostic testing are key factors propelling the adoption of Vaginitis Therapeutics. The trend towards comprehensive sexual health education and the increasing support from public health campaigns further contribute to market growth.
Restraint/Challenge:
“Emergence of Drug Resistance and Misdiagnosis”
- Concerns regarding the growing emergence of drug resistance in common vaginal pathogens, particularly among bacteria causing bacterial vaginosis and fungi causing candidiasis, coupled with the challenges of accurate self-diagnosis and potential for misdiagnosis, pose a significant challenge to broader market effectiveness and treatment outcomes
- For instance, a recent study highlighted an alarming increase in metronidazole-resistant bacterial vaginosis cases, raising anxieties among clinicians about limited treatment options. Addressing these resistance concerns through stewardship programs and the development of novel antimicrobial agents is crucial for maintaining therapeutic efficacy. Pharmaceutical companies are emphasizing the spectrum of activity and resistance profiles of new drugs in their marketing to reassure prescribers
- Additionally, the complexity of vaginitis symptoms, which can overlap among different types of infections or even non-infectious conditions, often leads to inappropriate self-treatment with OTC products or delayed professional diagnosis, resulting in recurrent infections or worsening symptoms
- While the potential for effective treatment exists, the growing resistance issues and diagnostic ambiguities can still hinder widespread resolution of conditions. Overcoming these challenges through enhanced diagnostic tools, improved patient education on seeking professional medical advice, and continued research into new drug targets will be vital for sustained market growth.
Vaginitis Therapeutics Market Scope
The market is segmented on the basis of type of vaginitis, drug class, route of administration, distribution channel, and end-user.
- By Type of Vaginitis
On the basis of type of vaginitis, the Vaginitis Therapeutics Market is segmented into Bacterial Vaginosis, Vulvovaginal Candidiasis (Yeast Infection), Trichomoniasis, Atrophic Vaginitis, and Other Vaginitis Types (e.g., Allergic Vaginitis). The Bacterial Vaginosis segment dominates the largest market revenue share of 43.2% in 2025, driven by its high prevalence globally, frequent recurrence, and the need for prescription antibiotic treatments. It is the most common cause of vaginal symptoms and requires specific therapeutic intervention. The Atrophic Vaginitis segment is anticipated to witness the fastest growth rate of 21.7% from 2025 to 2032, fueled by the aging global population and the increasing prevalence of menopause, which leads to estrogen deficiency and associated vaginal dryness and inflammation. The growing awareness and availability of hormonal and non-hormonal therapies for this condition drive its rapid growth.
- By Drug Class
On the basis of drug class, the Vaginitis Therapeutics Market is segmented into Antibiotics (e.g., Metronidazole, Clindamycin), Antifungals (e.g., Fluconazole, Miconazole), Antiprotozoals (e.g., Metronidazole, Tinidazole), Hormonal Therapies (e.g., Estrogen Creams), and Other Drugs (e.g., Anti-inflammatories). The Antibiotics (e.g., Metronidazole, Clindamycin) segment held the largest market revenue share in 2025, driven by their essential role in treating bacterial vaginosis and trichomoniasis, two of the most prevalent forms of vaginitis. These drugs are the frontline treatment for eradicating bacterial and parasitic causes. The Hormonal Therapies (e.g., Estrogen Creams) segment is expected to witness the fastest CAGR from 2025 to 2032, primarily due to the increasing aging female population and rising prevalence of menopausal symptoms leading to atrophic vaginitis. These therapies effectively address estrogen deficiency and relieve associated symptoms, driving significant demand.
- By Route of Administration
On the basis of route of administration, the Vaginitis Therapeutics Market is segmented into Oral, Topical (Creams, Gels, Suppositories), and Vaginal Rings. The Topical (Creams, Gels, Suppositories) segment held the largest market revenue share in 2025, driven by their direct action at the site of infection, minimizing systemic side effects, and offering targeted relief for various vaginitis types, including yeast infections and bacterial vaginosis. Their ease of use and immediate symptom relief contribute to their popularity. The Vaginal Rings segment is expected to witness the fastest CAGR from 2025 to 2032, primarily due to their convenience and sustained release of medication (especially hormonal therapies for atrophic vaginitis), providing long-term relief with less frequent application compared to daily creams or oral medications, thereby improving patient adherence.
- By Distribution Channel
On the basis of distribution channel, the Vaginitis Therapeutics Market is segmented into Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies. The Retail Pharmacies segment held the largest market revenue share in 2025, driven by their widespread accessibility, convenience for patients to purchase both prescription and over-the-counter vaginitis treatments, and their role as a primary point of contact for self-care advice. They serve a vast customer base for immediate treatment needs. The Online Pharmacies segment is expected to witness the fastest CAGR from 2025 to 2032, primarily due to the growing consumer preference for discretion, convenience, and competitive pricing when purchasing sensitive medications. The increasing adoption of e-commerce for healthcare products and the ability to offer home delivery fuels this segment's rapid growth.
- By End-User
On the basis of end-user, the Vaginitis Therapeutics Market is segmented into Hospitals, Gynecology Clinics, Primary Care Centers, and Homecare Settings. The Gynecology Clinics segment accounted for the largest market revenue share in 2024, driven by their specialized expertise in diagnosing, managing, and treating complex gynecological conditions, including various forms of vaginitis, often requiring specialized testing and tailored treatment plans. These clinics serve as referral centers for persistent or recurrent cases. The Homecare Settings segment is expected to witness the fastest CAGR from 2025 to 2032, driven by the increasing shift towards self-management of common vaginitis types using over-the-counter products, along with the growing adoption of telehealth services for remote consultations and prescription refills, allowing individuals to manage their conditions discreetly and conveniently.
Vaginitis Therapeutics Market Regional Analysis
North America dominates the Vaginitis Therapeutics Market with the largest revenue share of 40.01% in 2024, driven by a high prevalence of vaginal infections, advanced healthcare infrastructure that supports early diagnosis and treatment, and high patient awareness regarding reproductive health. The region benefits from significant pharmaceutical research and development, a robust regulatory framework, and strong access to both prescription and over-the-counter medications. This widespread adoption is further supported by proactive public health campaigns and a high level of patient engagement.
U.S. Vaginitis Therapeutics Market Insight
The U.S. Vaginitis Therapeutics Market captured the largest revenue share of 81.2% within North America in 2025, fueled by a high incidence of vaginitis, extensive research and development in women's health, and the presence of leading pharmaceutical companies. The country has a well-developed healthcare system with broad access to specialist gynecological care and a strong market for both prescription and OTC products.
Europe Vaginitis Therapeutics Market Insight
The European Vaginitis Therapeutics Market is projected to expand at a substantial CAGR throughout the forecast period, primarily driven by increasing awareness of women's health issues, improving diagnostic capabilities, and a rising focus on preventative care across various European countries. European regulatory bodies are increasingly supportive of new treatment options, and public health initiatives promote timely treatment.
U.K. Vaginitis Therapeutics Market Insight
The U.K. Vaginitis Therapeutics Market is anticipated to grow at a noteworthy CAGR during the forecast period, driven by increasing public health campaigns focused on sexual and reproductive health, a robust National Health Service (NHS) that provides accessible care, and a growing market for OTC treatments for common vaginal infections. The country also benefits from active research in gynecological conditions.
Germany Vaginitis Therapeutics Market Insight
The German Vaginitis Therapeutics Market is expected to expand at a considerable CAGR during the forecast period, fueled by its strong healthcare system, high patient awareness of women's health, and a growing emphasis on evidence-based treatment for various gynecological conditions. Germany's robust pharmaceutical industry and commitment to patient well-being contribute to market growth.
Asia-Pacific Vaginitis Therapeutics Market Insight
The Asia-Pacific Vaginitis Therapeutics Market is poised to grow at the fastest CAGR of over 24% in 2025, driven by increasing awareness of women's health, improving access to healthcare facilities, rising disposable incomes, and a large patient pool in countries such as China, Japan, and India. The expanding prevalence of vaginal infections and efforts to improve women's hygiene standards are key factors.
Japan Vaginitis Therapeutics Market Insight
The Japan Vaginitis Therapeutics Market is gaining momentum due to its technologically advanced healthcare system, high standards of personal hygiene, and a growing focus on women's reproductive health. The Japanese market benefits from a well-established pharmaceutical industry and a proactive approach to developing and adopting new treatments.
China Vaginitis Therapeutics Market Insight
The China Vaginitis Therapeutics Market accounted for the largest market revenue share in Asia Pacific in 2025, attributed to its vast population, increasing awareness of women's health issues, and expanding investments in healthcare infrastructure. Government initiatives to improve public health and a growing middle class seeking advanced medical solutions are key factors propelling the market in China.
Vaginitis Therapeutics Market Share
The Vaginitis Therapeutics industry is primarily led by well-established companies, including:
- Pfizer Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- Johnson & Johnson (U.S.)
- Sanofi (France)
- GlaxoSmithKline plc (U.K.)
- Bayer AG (Germany), Lupin Pharmaceuticals, Inc. (India),
- AstraZeneca (U.K.)
- Sanofi (France),
- Aimmune Therapeutics, Inc. (U.S.)
- DBV Technologies (France), Sanofi (France),
- Regeneron Pharmaceuticals, Inc. (U.S.)
- Pfizer Inc. (U.S.)
- Johnson & Johnson (U.S.)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Bayer AG (Germany),
- Mylan N.V. (U.S.)
- Kaléo, Inc. (U.S.)
- Camallergy (U.K.)
- Astellas Pharma Inc. (Japan)
- Novartis AG (Switzerland)
- Allergy Therapeutics plc (U.K.)
- Genentech, Inc. (U.S.)
- Merck & Co., Inc. (U.S.)
- Viatris Inc. (U.S.),
- ALK-Abelló A/S (Denmark)
- Leti Pharma (Spain)
- Stallergenes Greer (U.K.)
Latest Developments in Global Vaginitis Therapeutics Market
- In April 2023, Mycovia Pharmaceuticals, a company focused on women's health, announced the launch of its novel oral antifungal treatment for recurrent vulvovaginal candidiasis, offering a new option for patients with chronic yeast infections. This development aims to provide a more convenient and effective solution for recurring infections
- In March 2023, TherapeuticsMD, Inc., a women's health company, reported positive Phase 3 clinical trial results for a new non-antibiotic treatment for bacterial vaginosis, signaling potential for an alternative approach to managing this common condition. This advancement aims to reduce reliance on antibiotics and address resistance concerns
- In March 2023, Dare Bioscience, a biopharmaceutical company, announced a strategic partnership with a global pharmaceutical firm to accelerate the development and commercialization of its investigational intravaginal ring for bacterial vaginosis, aiming to provide a long-acting, patient-friendly therapeutic option. This collaboration is expected to improve product reach and patient adherence
- In February 2023, Shionogi Inc., a Japanese pharmaceutical company, received FDA approval for a new antibiotic for trichomoniasis with a simplified dosing regimen, offering a more convenient treatment option for patients and healthcare providers. This approval is expected to improve patient compliance
- In January 2023, Sebela Pharmaceuticals, Inc., a specialty pharmaceutical company, launched a new line of non-hormonal vaginal moisturizers and lubricants specifically designed for atrophic vaginitis, catering to patients who cannot use or prefer not to use estrogen-based therapies. This expansion offers a wider range of solutions for atrophic vaginitis management
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.