Global Vasomotor Menopausal Symptoms Vms Treatment Market
Market Size in USD Billion
CAGR :
% 
 USD
18.85 Billion
USD
30.50 Billion
2024
2032
USD
18.85 Billion
USD
30.50 Billion
2024
2032
| 2025 –2032 | |
| USD 18.85 Billion | |
| USD 30.50 Billion | |
|
|
|
|
Vasomotor Menopausal Symptoms (VMS) Treatment Market Size
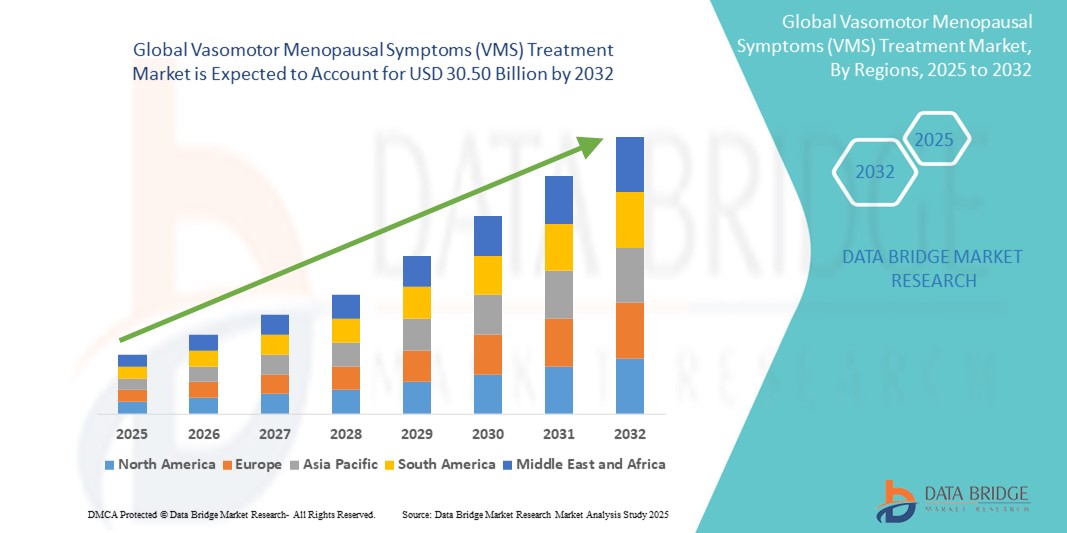
- The global vasomotor menopausal symptoms (VMS) treatment market size was valued at USD 18.85 billion in 2024 and is expected to reach USD 30.50 billion by 2032, at a CAGR of 6.20% during the forecast period
- The market growth is largely fuelled by the rising awareness and adoption of hormone and non-hormone therapies for managing vasomotor menopausal symptoms, including hot flashes and night sweats, which significantly impact the quality of life in menopausal women. This has led to increased demand for both prescription-based and over-the-counter treatment options across various geographies
- Furthermore, increasing consumer preference for safe, effective, and personalized treatment alternatives—such as hormone replacement therapy (HRT), selective estrogen receptor modulators (SERMs), and neurokinin-3 receptor (NK3R) antagonists—is establishing VMS treatment as a critical segment in women's healthcare. These converging factors are accelerating the uptake of Vasomotor Menopausal Symptoms (VMS) Treatment solutions, thereby significantly boosting the industry's growth
Vasomotor Menopausal Symptoms (VMS) Treatment Market Analysis
- Vasomotor Menopausal Symptoms (VMS) treatments, including hormone therapy and non-hormonal options, are becoming essential components of modern women’s health management, especially in addressing symptoms such as hot flashes and night sweats. Their increasing clinical validation, convenience, and patient acceptance are expanding their role in both prescription and over-the-counter healthcare settings
- The escalating demand for VMS treatments is primarily fueled by the growing global menopausal population, rising awareness of menopause management options, and increasing investments in women’s health by pharmaceutical companies. The availability of newer, non-hormonal therapies such as NK3 receptor antagonists is also expected to reshape treatment preferences
- North America dominated the vasomotor menopausal symptoms (VMS) treatment market, holding the largest revenue share of 42.3% in 2024, driven by early diagnosis practices, widespread awareness, strong healthcare infrastructure, and a high adoption rate of both hormonal and non-hormonal therapies
- Asia-Pacific is expected to be the fastest-growing region in the vasomotor menopausal symptoms (VMS) treatment market, projected to expand at a CAGR of 9.8%, owing to increasing urbanization, growing female geriatric population, and rising awareness of menopause-related health solutions
- The hormone therapy segment dominated the vasomotor menopausal symptoms (VMS) treatment market with a market share of 45.7% in 2024, supported by its long-established efficacy in relieving VMS and growing endorsement by healthcare professionals
Report Scope and Vasomotor Menopausal Symptoms (VMS) Treatment Market Segmentation
|
Attributes |
Vasomotor Menopausal Symptoms (VMS) Treatment Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Vasomotor Menopausal Symptoms (VMS) Treatment Market Trends
“Innovative Delivery Methods and Digital Therapeutics Gaining Traction”
- A significant and accelerating trend in the global vasomotor menopausal symptoms (VMS) Treatment market is the growing emphasis on innovative drug delivery systems and digital therapeutic platforms. These advancements are enhancing treatment adherence, patient comfort, and therapeutic outcomes
- For instance, Bayer AG has been actively investing in transdermal delivery patches for estrogen therapy, which offer a non-invasive, easy-to-use alternative to oral hormone therapies. These patches provide consistent hormone levels, reduced gastrointestinal side effects, and improved patient compliance
- Similarly, companies such as Theramex and Astellas Pharma are focusing on oral neurokinin-3 receptor antagonists, such as fezolinetant, which address VMS without the risks associated with hormone replacement therapy. This innovation is expanding treatment options for women who are contraindicated or unwilling to use hormonal intervention
- In parallel, digital therapeutics and mobile health platforms are emerging as adjunct solutions to manage VMS symptoms through cognitive behavioral therapy (CBT), lifestyle tracking, and personalized treatment plans. Platforms such as Mayo Clinic’s My Menopause Doctor and Lisa Health’s Midday app enable users to log symptoms, receive evidence-based recommendations, and track progress over time
- The integration of mobile apps with wearable devices also allows real-time monitoring of sleep patterns, hot flash frequency, and mood fluctuations, empowering patients and clinicians with actionable data for optimized care
- This trend towards user-friendly, non-invasive, and digitally supported therapies is fundamentally reshaping the management of menopausal symptoms. As demand grows for personalized and holistic VMS treatments, pharmaceutical and digital health companies are increasingly collaborating to develop multimodal, accessible, and data-driven solutions for menopausal women globally
Vasomotor Menopausal Symptoms (VMS) Treatment Market Dynamics
Driver
“Growing Need Due to Rising Awareness and Expanding Postmenopausal Population”
- The increasing prevalence of menopausal symptoms among aging women, particularly hot flashes and night sweats, coupled with rising awareness and medical consultation rates, is a significant driver for the heightened demand for VMS treatment options
- For instance, in January 2024, Astellas Pharma Inc. received FDA approval for Veozah (fezolinetant), a non-hormonal neurokinin-3 receptor antagonist designed specifically to treat moderate to severe VMS. Such strategic innovations by key companies are expected to drive the VMS Treatment industry growth in the forecast periods
- As women seek safer and more effective alternatives to hormone replacement therapy (HRT), the demand for non-hormonal and targeted therapies is rising steadily. These solutions offer symptom relief without the risks associated with estrogen-based therapies
- Furthermore, the growing focus on personalized medicine, combined with increasing investments in women’s health by public and private stakeholders, is fostering innovation and improved treatment accessibility for VMS sufferers
- The availability of multiple delivery options including oral, transdermal, and extended-release formats, as well as emerging digital health platforms that monitor symptoms and enhance treatment adherence, further contribute to the market’s sustained growth
Restraint/Challenge
“Safety Concerns and Limited Accessibility of Advanced Therapies”
- Despite increasing therapeutic options, safety concerns regarding hormonal treatments and the limited awareness or availability of non-hormonal alternatives in low- and middle-income countries pose major barriers to broader VMS treatment adoption
- For instance, studies have linked long-term HRT use with increased risks of breast cancer and cardiovascular complications, making patients and providers more cautious about initiating or continuing these therapies
- Addressing these concerns through transparent labeling, continued clinical trials, and real-world safety data will be essential for improving patient trust and expanding uptake
- In addition, high costs associated with newly approved non-hormonal treatments, such as fezolinetant, may restrict access among uninsured or underinsured populations
- While generic and OTC products provide some relief, they may lack the efficacy of prescription therapies. Thus, equitable access and reimbursement support are critical to avoid disparities in menopause care
Vasomotor Menopausal Symptoms (VMS) Treatment Market Scope
The market is segmented on the basis of drug class, route of administration, end-users, and distribution channel.
• By Drug Class
On the basis of drug class, the vasomotor menopausal symptoms (VMS) treatment market is segmented into antidepressants, hormone therapy, anticonvulsants, and others. Hormone therapy dominated the market with the largest revenue share of 47.3% in 2024, owing to its high efficacy in managing severe menopausal symptoms such as hot flashes and night sweats.
Antidepressants are expected to witness the fastest CAGR of 10.8% from 2025 to 2032, driven by increasing demand for non-hormonal alternatives due to safety concerns related to hormone therapy.
• By Route of Administration
On the basis of route of administration, the vasomotor menopausal symptoms (VMS) treatment market is segmented into oral, parenteral, and others. The oral segment held the largest revenue share of 62.5% in 2024, due to ease of administration, patient compliance, and the popularity of tablets and capsules.
The parenteral segment is projected to grow at the fastest CAGR of 9.6% during 2025–2032, as long-acting injectables and hormone patches gain traction for their sustained release and convenience.
• By End-Users
On the basis of end-users, the vasomotor menopausal symptoms (VMS) treatment market is segmented into hospitals, specialty clinics, homecare, and others. The Hospitals segment accounted for the largest market share of 38.2% in 2024, attributed to comprehensive care, physician guidance, and availability of advanced diagnostic and treatment tools.
The homecare segment is expected to expand at the fastest CAGR of 11.2% from 2025 to 2032, supported by increasing telehealth services, patient comfort, and rising home-use therapy adoption.
• By Distribution Channel
On the basis of distribution channel, the vasomotor menopausal symptoms (VMS) treatment market is segmented into hospital pharmacy, retail pharmacy, online pharmacy, and others. The retail pharmacy segment led the market with the highest share of 44.7% in 2024, due to widespread availability and convenience in prescription fulfillment.
The online pharmacy segment is projected to record the fastest CAGR of 13.4% during the forecast period, driven by e-commerce growth, digital health adoption, and preference for doorstep medication delivery.
Vasomotor Menopausal Symptoms (VMS) Treatment Market Regional Analysis
- North America dominated the vasomotor menopausal symptoms (VMS) treatment market with the largest revenue share of 42.3% in 2024, driven by the high prevalence of menopause-related conditions, rising awareness regarding treatment options, and the availability of advanced hormone and non-hormone therapies
- The region benefits from a well-established healthcare infrastructure, favorable reimbursement policies, and proactive initiatives to address women’s health issues
- In addition, strong pharmaceutical R&D investments, robust clinical trial activity, and widespread access to both prescription and over-the-counter VMS treatments contribute to the region’s leading position in the global market
U.S. Vasomotor Menopausal Symptoms (VMS) Treatment Market Insight
The U.S. vasomotor menopausal symptoms (VMS) treatment market captured the largest revenue share of 71% in 2024 within North America, driven by the high prevalence of menopause-related symptoms and widespread availability of hormone and non-hormone therapies. Strong healthcare infrastructure, high awareness levels, and aggressive pharmaceutical R&D spending by major players such as Pfizer and AbbVie are accelerating treatment uptake. The presence of reimbursement policies, broad access to prescription medications, and the rising demand for personalized treatment options are key growth enablers in the U.S. market.
Europe Vasomotor Menopausal Symptoms (VMS) Treatment Market Insight
The Europe vasomotor menopausal symptoms (VMS) treatment market held a significant revenue share of 28.7% of the global market in 2024. Market expansion is supported by increasing healthcare expenditure, a growing aging female population, and supportive regulatory frameworks for hormone therapy. Countries such as Germany, the U.K., and France are leading contributors, driven by higher awareness, access to specialists, and active research into hormone and non-hormone alternatives. The region is also witnessing growing demand for OTC solutions and non-estrogen therapies.
U.K. Vasomotor Menopausal Symptoms (VMS) Treatment Market Insight
The U.K. vasomotor menopausal symptoms (VMS) treatment market accounted for 21.4% of the European market share in 2024. The market is benefiting from strong NHS initiatives to improve women's health services and ongoing clinical trials for non-hormonal therapies. Rising awareness about menopausal symptoms and increasing access to over-the-counter options are key drivers. Government policies promoting early intervention and public campaigns have helped normalize menopausal health conversations, thus boosting diagnosis and treatment rates.
Germany Vasomotor Menopausal Symptoms (VMS) Treatment Market Insight
The Germany vasomotor menopausal symptoms (VMS) treatment captured the largest share of 26.8% in 2024 within Europe. Growth in Germany is supported by high per capita healthcare spending, active participation of pharmaceutical companies in menopause-related R&D, and well-structured insurance systems that ensure treatment access. The country is a hub for innovation in hormone therapy formulations and personalized medicine approaches targeting vasomotor symptoms.
Asia-Pacific Vasomotor Menopausal Symptoms (VMS) Treatment Market Insight
The Asia-Pacific vasomotor menopausal symptoms (VMS) treatment market is projected to grow at the fastest CAGR of 9.8% during the forecast period (2025–2032). Rising awareness of women’s health, increasing healthcare investments, and improving diagnostic services are key drivers. Countries such as China, Japan, and India are witnessing significant growth due to expanding middle-class populations, higher female life expectancy, and increased access to both traditional and modern menopause treatments.
China Vasomotor Menopausal Symptoms (VMS) Treatment Market Insight
The China vasomotor menopausal symptoms (VMS) treatment led the Asia-Pacific VMS Treatment market with a 45.7% share in 2024. The market is expanding due to large population size, rising incidence of menopause-related symptoms, and increased government support for women’s health. Growing adoption of OTC medications, local pharmaceutical development, and urbanization are accelerating demand. Moreover, the shift toward wellness and preventive healthcare is opening new opportunities for herbal and non-hormonal alternatives.
India Vasomotor Menopausal Symptoms (VMS) Treatment Market Insight
The India vasomotor menopausal symptoms (VMS) treatment accounted for 16.1% of the Asia-Pacific VMS Treatment market in 2024 and is projected to grow at the fastest CAGR of 26.9% through 2032. Market expansion is driven by growing awareness of menopausal health, an increasing number of specialty clinics, and rising urban female population. Local pharmaceutical manufacturers are entering the space with cost-effective hormone therapy options and OTC supplements. Government-led women’s health initiatives and the spread of digital health platforms are also improving treatment access and education, especially in tier-2 and tier-3 cities.
Vasomotor Menopausal Symptoms (VMS) Treatment Market Share
The vasomotor menopausal symptoms (VMS) treatment industry is primarily led by well-established companies, including:
- Zhejiang Huahai Pharmaceutical Co., Ltd (China)
- Teva Pharmaceutical Industries Ltd. (Israel)
- Pfizer Inc. (U.S.)
- Hikma Pharmaceutical PLC (U.K.)
- Amneal Pharmaceuticals LLC. (U.S.)
- Alembic Pharmaceuticals Limited (India)
- Zydus Group (India)
- Dr. Reddy’s Laboratories Ltd (India)
- Abbvie, Inc. (U.S.)
- Sun Pharmaceutical Industries Ltd (India)
- Apotex Inc. (Canada)
- Aurobindo Pharma (India)
- Endo Pharmaceuticals plc (Ireland)
- Lupin (India)
- Novartis AG (Switzerland)
- WOCKHARDT (India)
Latest Developments in Global Vasomotor Menopausal Symptoms (VMS) Treatment Market
- In May 2023, Astellas Pharma Inc. achieved a significant milestone with the FDA approval of VEOZAH (fezolinetant), making it the first non-hormonal neurokinin 3 (NK3) receptor antagonist specifically approved for the treatment of moderate to severe VMS associated with menopause. This oral, once-daily medication offers a novel mechanism of action, addressing a critical unmet need for women who cannot or prefer not to use hormonal therapies. Clinical trials (SKYLIGHT 1 and SKYLIGHT 2) demonstrated its efficacy in significantly reducing the frequency and severity of hot flashes
- In March 2023, The Lancet published results from Astellas' pivotal Phase 3 SKYLIGHT 1™ study of fezolinetant, further solidifying its efficacy and safety profile for VMS treatment. The successful publication of such robust clinical data provides crucial evidence supporting the adoption of new non-hormonal options by healthcare providers and patients, influencing market dynamics
- In June 2023, Astellas Pharma Inc. announced positive topline results from the Phase 3b DAYLIGHT clinical trial for fezolinetant. This study specifically evaluated the 24-week efficacy and safety in more than 450 women considered unsuitable for hormone therapy, showing a statistically significant reduction in VMS frequency. This further expands the evidence base for fezolinetant, addressing a specific patient population within the VMS market
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.