Global Vectorized Antibodies For In Vivo Expression Market
Market Size in USD Billion
CAGR :
% 
 USD
1.35 Billion
USD
2.54 Billion
2024
2032
USD
1.35 Billion
USD
2.54 Billion
2024
2032
| 2025 –2032 | |
| USD 1.35 Billion | |
| USD 2.54 Billion | |
|
|
|
|
Vectorized Antibodies for In Vivo Expression Market Size
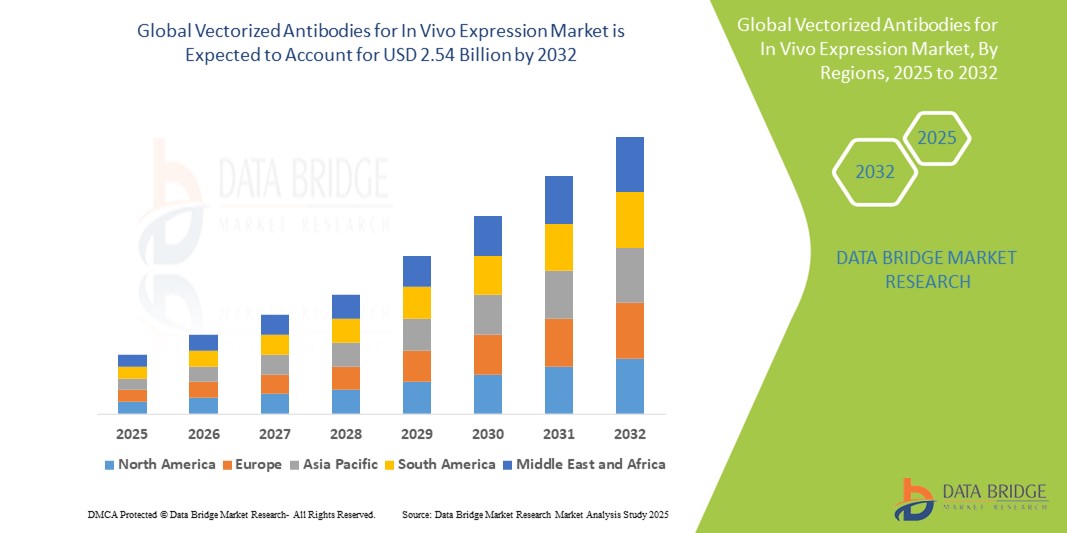
- The global vectorized antibodies for in vivo expression market was valued at USD 1.35 billion in 2024 and is expected to reach USD 2.54 billion by 2032
- During the forecast period of 2025 to 2032 the market is likely to grow at a CAGR of 8.20% primarily driven by the increasing demand and therapeutic potential
- This growth is driven by factors such as the rising prevalence of chronic diseases and advancements in gene delivery technologies
Vectorized Antibodies for In Vivo Expression Market Analysis
- The market for vectorized antibodies is gaining strong momentum, with companies such as Regeneron and Moderna actively developing in vivo expression platforms. These platforms allow the body to produce therapeutic antibodies internally, reducing the need for repeated injections or infusions
- Advanced delivery methods such as adeno-associated virus vectors and lipid nanoparticles are being applied by companies such as Spark Therapeutics and BioNTech
- For instance, BioNTech is exploring lipid nanoparticle-based delivery systems to enable the body to generate its own antibodies against cancer cells
- Real-world research instance has demonstrated the potential of this approach
- For instance, the University of Pennsylvania conducted a study in which a single administration of a vectorized antibody led to sustained therapeutic levels in animal models for hemophilia and certain cancers
- Collaborative initiatives are playing a major role in accelerating innovation
- For instance, the University of California partnered with Genentech to co-develop long-lasting and less invasive treatments for autoimmune and neurological diseases using in vivo antibody expression technologies
- Regulatory agencies are increasingly supportive of these developments
- For instance, the U.S. Food and Drug Administration has recently approved investigational new drug applications submitted by biotechnology companies for experimental vectorized antibody treatments targeting rare pediatric conditions
Report Scope and Vectorized Antibodies for In Vivo Expression Market Segmentation
|
Attributes |
Vectorized Antibodies for In Vivo Expression Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Vectorized Antibodies for In Vivo Expression Market Trends
“Growing Collaboration between Biotechnology Companies, Research Institutions, and Healthcare Providers”
- The increasing collaboration between biotechnology companies, research institutions, and healthcare providers is fostering faster development of novel therapies, particularly in the field of vectorized antibodies for in vivo expression
- For instance, companies such as Genentech have teamed up with universities such as Stanford to develop new antibody therapies
- Biotechnology firms such as Moderna and Novavax have partnered with research institutions to leverage advanced gene delivery technologies, accelerating the creation of new treatments. Moderna
- For instance, collaborated with the University of Maryland for its COVID-19 vaccine development, showcasing how such partnerships can lead to breakthrough innovations
- Research institutions such as the National Institutes of Health (NIH) are instrumental in discovering innovative methods for delivering therapeutic antibodies. NIH has worked closely with biotech firms such as Intellia Therapeutics to develop CRISPR-based gene editing therapies, which have promising applications for in vivo antibody delivery
- Healthcare providers play a critical role in the practical application and testing of these technologies, with hospitals and clinical centers participating in trials to bring therapies to market more efficiently. Hospitals such as Mayo Clinic have collaborated with pharmaceutical companies to test new antibody therapies in clinical trials, directly influencing treatment options
- Such collaborations are not only streamlining research and development but are also helping to navigate regulatory challenges
- For instance, biotech companies such as Regeneron have worked with the U.S. Food and Drug Administration (FDA) to fast-track the approval of their monoclonal antibody therapies for COVID-19
Vectorized Antibodies for In Vivo Expression Market Dynamics
Driver
“Advancements in Gene Delivery Technologies”
- Recent breakthroughs in gene delivery technologies have improved the delivery efficiency of vectorized antibodies for in vivo expression, allowing for more precise and effective therapies
- For instance, the use of adeno-associated virus vectors has been instrumental in delivering therapeutic genes for conditions such as spinal muscular atrophy, improving patient outcomes significantly
- Innovations such as adeno-associated virus vectors, which are used in gene therapies such as those for spinal muscular atrophy, have enhanced the ability to deliver antibodies directly to specific tissues or cells
- For instance, the approval of Zolgensma, a gene therapy that utilizes AAV vectors to treat spinal muscular atrophy, demonstrating the potential of these technologies in clinical settings
- Lipid nanoparticles, which played a crucial role in the delivery of mRNA vaccines such as the Pfizer-BioNTech and Moderna COVID-19 vaccines, have been further optimized for better stability and efficiency in transporting therapeutic antibodies. These nanoparticles have shown promising results in efficiently delivering mRNA-based therapies, making them a key player in the future of vectorized antibody delivery
- Electroporation techniques, which use electrical fields to enhance cell membrane permeability, have been shown to improve the delivery of plasmid DNA and antibody-encoding genes
- For instance, electroporation has been used successfully in clinical trials for cancer therapies, enhancing the delivery of therapeutic antibodies directly into tumor cells, thus improving the effectiveness of cancer treatments
- These advancements are enabling broader applications across complex diseases, such as gene-based treatments for Duchenne muscular dystrophy and targeted cancer therapies such as those used in CAR-T cell treatments
- For instance, the use of vectorized antibodies in CAR-T cell therapies has shown promise in treating blood cancers such as leukemia, where the antibody helps target and destroy cancer cells effectively
Opportunity
“Expansion into Rare and Complex Diseases”
- Vectorized antibody therapies present a significant opportunity for treating rare and complex diseases that lack effective treatment options
- For instance, gene therapies utilizing vectorized antibodies have shown promise in treating rare genetic disorders, where traditional therapies have been ineffective
- By enabling the targeted delivery of therapeutic agents directly to disease sites, these therapies offer the potential to address conditions such as Duchenne muscular dystrophy, where corrective genes can be delivered to specific tissues
- For instance, this is the clinical trial conducted by Sarepta Therapeutics, which is testing gene therapies to treat Duchenne muscular dystrophy using vectorized antibodies
- These therapies can also be applied to rare cancers, where conventional treatments such as chemotherapy and radiation often have limited effectiveness
- For instance, in clinical trials for rare blood cancers such as acute lymphoblastic leukemia, CAR-T cell therapies have successfully used vectorized antibodies to target and destroy cancer cells directly, offering hope to patients with few treatments’ options
- The targeted approach of vectorized antibody therapies not only improves therapeutic outcomes but also reduces potential side effects
- For instance, the use of targeted gene therapy for cystic fibrosis, where vectorized delivery methods ensure that therapeutic genes reach the lungs with minimal systemic side effects
- This innovative strategy of directly targeting disease sites makes vectorized antibody therapies a valuable tool in treating conditions that have been challenging to manage with conventional therapies, opening up new avenues for treatment of previously untreatable diseases such as certain genetic disorders and rare cancers
Restraint/Challenge
“High Development and Production Costs”
- The development and production of vectorized antibodies involve complex and resource-intensive processes, which lead to high costs
- For instance, the production of gene therapies requires specialized facilities for the handling and preparation of viral vectors, adding significant overhead to the development process
- Manufacturing these therapies requires skilled personnel with expertise in areas such as molecular biology, genetics, and bioengineering
- For instance, of this is seen in companies such as Moderna, where a team of experts is needed to synthesize and scale up mRNA therapies for global distribution, significantly increasing production costs
- Adherence to stringent regulatory standards, such as those set by the U.S. Food and Drug Administration (FDA), is essential to ensure the safety and efficacy of these therapies
- For instance, the rigorous quality control and testing procedures required for approval of mRNA vaccines, such as those from Pfizer-BioNTech, add to the overall expense of production
- The production of mRNA-based therapies necessitates precise synthesis and quality control measures to ensure efficacy and safety. This includes maintaining the stability of lipid nanoparticles and ensuring that the mRNA strands are properly encoded and delivered, a process that requires advanced technology and significant investment
- These high costs can limit accessibility and affordability, particularly in low-resource settings, posing a challenge to the widespread adoption of vectorized antibody therapies. The high price tag of treatments such as gene therapy for rare diseases, which can exceed millions of dollars per patient, raises concerns about their availability to a broader patient population
Vectorized Antibodies for In Vivo Expression Market Scope
The market is segmented on the basis of technology and end user
|
Segmentation |
Sub-Segmentation |
|
By Technology |
|
|
By End User |
|
Vectorized Antibodies for In Vivo Expression Market Regional Analysis
“North America is the Dominant Region in the Vectorized Antibodies for In Vivo Expression Market”
- North America is the dominant region in the vectorized antibodies for in vivo expression market, benefiting from a strong biotechnology infrastructure that supports innovation and development
- The U.S. plays a central role, with numerous advanced research institutions and leading biopharmaceutical companies driving progress in genetic therapies and antibody-based treatments
- A strong healthcare system in North America, coupled with a supportive regulatory environment, fosters the adoption and commercialization of cutting-edge therapies such as vectorized antibodies
- Continuous investment in research and development ensures that North America remains at the forefront of biotechnology and gene therapy advancements
- The high demand for personalized medicine and targeted treatment options in the region further solidifies North America's position as a global leader in the development of vectorized antibody therapies
“Asia-Pacific is Projected to Register the Highest Growth Rate”
- Asia Pacific is the fastest-growing region in the market for vectorized antibodies for in vivo expression, driven by rapid advancements in biotechnology research
- Countries such as China and India are leading the charge, with significant investments in biotechnology and an increase in healthcare spending to support innovation
- Government support for research and development, alongside rising awareness of advanced treatments such as gene therapies, is propelling the market’s growth in the region
- The increasing prevalence of chronic diseases and growing demand for targeted therapies further contribute to the accelerated expansion of the market in Asia Pacific
- The region's emerging biotechnology sector, along with efforts to improve healthcare infrastructure, is facilitating the faster adoption of innovative therapies, positioning Asia Pacific as a key player in biopharmaceutical advancements
Vectorized Antibodies for In Vivo Expression Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- 4D Molecular Therapeutics (U.S.)
- AbbVie (U.S.)
- Adverum Biotechnologies (U.S.)
- AstraZeneca (U.K.)
- BioNTech (Germany)
- CureVac (Germany)
- Eli Lilly (U.S.)
- Ethris (Germany)
- Eyevensys (France)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.