Global Vegf B Inhibitors Market
Market Size in USD Billion
CAGR :
% 
 USD
1.27 Billion
USD
3.75 Billion
2024
2032
USD
1.27 Billion
USD
3.75 Billion
2024
2032
| 2025 –2032 | |
| USD 1.27 Billion | |
| USD 3.75 Billion | |
|
|
|
|
Global VEGF-B Inhibitors Market Analysis
VEGF-B Inhibitors is a rapidly growing segment within the broader oncology and drug development landscape, driven by the increasing recognition of vascular endothelial growth factor-C (VEGF-C) as a key factor in the development of lymphatic vessels and the progression of various cancers. VEGF-C plays a critical role in the lymphangiogenesis process, contributing to tumor growth, metastasis, and resistance to therapies. As such, VEGF-C inhibitors are being explored as a promising therapeutic approach for treating cancers like breast cancer, melanoma, and non-small cell lung cancer (NSCLC), where lymphatic spread is a significant concern.
Global VEGF-B Inhibitors Market Size
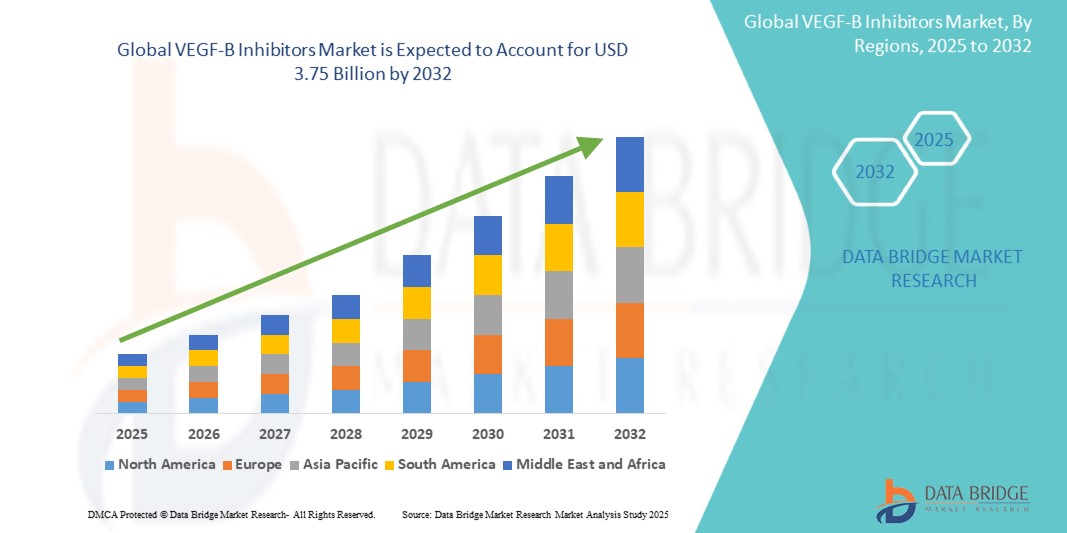
Global VEGF-B inhibitors market size was valued at USD 1.27 Billion in 2024 and is projected to reach USD 3.75 billion by 2032, with a CAGR of 14.4% during the forecast period of 2025 to 2032. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Global VEGF-B Inhibitors Market Trends
“Shift Toward Non-invasive Solutions”
The shift toward non-invasive solutions in the VEGF-B inhibitors market is becoming more noticeable as patients increasingly opt for procedures that involve less risk and faster recovery times. Endoscopic procedures and gastric balloons are gaining popularity due to their minimally invasive nature, offering effective weight loss results with no need for surgical incisions. These methods are seen as appealing alternatives to traditional bariatric surgeries like gastric bypass or sleeve gastrectomy, which involve more complex operations and longer recovery periods.
Non-surgical options are often associated with lower complication rates, fewer hospital stays, and quicker returns to normal activities. This trend reflects a growing preference for treatments that prioritize convenience and safety, particularly for patients hesitant about undergoing more invasive procedures. As awareness about these options spreads, more individuals are choosing non-invasive treatments to manage obesity effectively.
Report Scope and Global VEGF-B Inhibitors Market Segmentation
|
Attributes |
Global Bariatric Medical Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa, Brazil, Argentina, Rest of South America |
|
Key Market Players |
Roche/Genentech (Switzerland/U.S.), Regeneron Pharmaceuticals (U.S.), Novartis AG (Switzerland), Pfizer Inc. (U.S.), Eli Lilly and Company (U.S.), Bayer AG (Germany), Amgen (U.S.), Biocon (India), Samsung Bioepis (South Korea), and Alcon (Switzerland) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Global VEGF-B Inhibitors Market Definition
The VEGF-B inhibitors block vascular endothelial growth factor-B (VEGF-B), a protein involved in angiogenesis and lipid metabolism. By preventing VEGF-B from binding to its receptors, these inhibitors disrupt vascular growth and fatty acid transport. They are being explored as potential treatments for cancer, diabetic complications, cardiovascular diseases, and metabolic disorders, with ongoing research into their therapeutic applications.
Global VEGF-B Inhibitors Market Dynamics
Drivers
- Innovative Drug Developments
Innovative drug developments in anti-VEGF therapies, particularly through biosimilars and combination therapies, are driving significant advancements in the market. The introduction of biosimilars, such as Mvasi (a biosimilar of Bevacizumab), has improved accessibility to anti-VEGF treatments by providing cost-effective alternatives to expensive branded biologics. This development is particularly beneficial for patients in low and middle-income countries. The global biosimilars market is experiencing rapid growth, reflecting a growing demand for affordable biologics that help reduce treatment costs for conditions like age-related macular degeneration (AMD), diabetic retinopathy, and cancers. The synergy between these therapies, where the anti-VEGF agent blocks blood vessel growth that supports tumors and chemotherapy targets cancer cells directly, has proven to be particularly effective in treating non-small cell lung cancer (NSCLC), colorectal cancer, and renal cell carcinoma. This approach has significantly improved patient survival rates, leading to the widespread adoption of combination therapies in cancer treatment regimens.
- Expanding Indications for Anti-VEGF Therapies
The application of anti-VEGF therapies is expanding beyond their initial use in ophthalmology and oncology to address a variety of other medical conditions, including cardiovascular diseases and inflammatory disorders. In cardiovascular conditions, particularly ischemic heart diseases, where abnormal vascular growth and endothelial dysfunction contribute to disease progression, anti-VEGF treatments are being explored for their potential to prevent or treat conditions like heart failure, stroke, and other vascular complications. Similarly, in inflammatory disorders such as rheumatoid arthritis and psoriasis, where excessive blood vessel growth contributes to tissue inflammation, anti-VEGF therapies are under investigation as a potential treatment. In 2020, Bevacizumab alone generated over USD 7 billion in global sales, underlining its significance in cancer treatment. The success of Bevacizumab has spurred the development of additional anti-VEGF agents and combination therapies aimed at maximizing treatment outcomes, further fueling the growth and diversification of the anti-VEGF therapy market.
Opportunities
- Emerging Markets in the Asia-Pacific Region
Emerging markets, particularly in the Asia-Pacific region, present a significant opportunity for the growth of VEGF inhibitors. This region is experiencing rapid expansion in its healthcare sector, supported by substantial investments in healthcare infrastructure and the increasing availability of advanced medical treatments. The Asia-Pacific healthcare market, reflects the rising demand for innovative therapies as access to healthcare improves. In middle-income countries within this region, the growing awareness of VEGF-related conditions, such as cancer and retinal diseases, is driving the adoption of treatments like Bevacizumab and Ranibizumab. These therapies, which are crucial for managing conditions associated with abnormal blood vessel growth, are becoming more accessible due to improved healthcare facilities and resources. As a result, the Asia-Pacific region is poised to become a key growth driver for the VEGF inhibitor market, offering opportunities for both established players and new entrants to expand their presence and address the unmet needs of a rapidly growing patient population.
- Advancements in Personalized Medicine
Advancements in personalized medicine represent a significant opportunity for enhancing the efficacy of VEGF inhibitors, particularly in oncology. The shift toward biomarker-driven therapies is allowing for more customized treatments that target the specific genetic and molecular profiles of individual patients. This approach is expected to improve outcomes by ensuring that only those patients who are most likely to benefit from therapies like Bevacizumab receive them, thus enhancing the therapeutic effectiveness while minimizing potential side effects. For example, identifying genetic markers that predict a positive response to Bevacizumab can optimize its use, ensuring better patient outcomes and more efficient treatment regimens. Beyond oncology, precision medicine is also expanding the application of VEGF-B inhibitors, not just in cancer treatment but also in cardiovascular diseases and inflammatory disorders. By tailoring treatments based on individual patient needs, personalized medicine is helping to unlock new therapeutic possibilities, offering more targeted and effective solutions for a wide range of conditions. This evolution towards precision-based therapies is a key factor in the growing adoption of VEGF inhibitors and is expected to drive further market growth as it continues to expand into other therapeutic areas.
Restraints/Challenges
- High Therapy Costs
High therapy costs pose a significant restraint on the accessibility and widespread adoption of VEGF inhibitors, particularly in regions with limited healthcare budgets. The premium pricing of branded biologics, such as Bevacizumab (Avastin) and Eylea, creates substantial financial barriers for both patients and healthcare systems. For instance, the cost of a single monthly treatment cycle with Bevacizumab can exceed $10,000, making it unaffordable for many patients, especially in lower-income regions and middle-income countries. This high cost often restricts access to life-saving treatments, leaving a significant portion of the patient population untreated. Additionally, the financial strain on healthcare systems is considerable, as they face the challenge of balancing budget constraints with the need to provide effective therapies. The overall expense associated with VEGF inhibitor treatments highlights the critical need for cost-effective alternatives, such as biosimilars, and innovative pricing models to ensure that these therapies are accessible to a broader population while maintaining the sustainability of healthcare spending.
- Stringent Regulatory Pathways
Stringent regulatory pathways represent a significant restraint in the development and commercialization of VEGF inhibitors and their biosimilars. The approval process for new therapies and biosimilars is both lengthy and complex, requiring manufacturers to meet rigorous standards set by regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). These agencies demand extensive clinical evidence to demonstrate the safety, efficacy, and comparability of biosimilars to their reference products. This meticulous process, while essential for ensuring patient safety and treatment effectiveness, often results in delayed market entry, limiting the availability of cost-effective alternatives in the short term. Furthermore, the high costs associated with meeting these stringent regulatory requirements can deter smaller companies from entering the market, reducing competition and innovation. For established players, these challenges increase the time and financial investment needed to bring new products to market, ultimately affecting the affordability and accessibility of VEGF inhibitors for patients worldwide.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Global VEGF-B Inhibitors Market Scope
The market is segmented on the basis of drug class, therapeutic application, route of administration, and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Drug Class
- Anti-VEGF Monoclonal Antibodies
- VEGF-B Inhibitors
- Small Molecule Inhibitors
Therapeutic Application
- Oncology
- Ophthalmology
- Other Disorders
Route of Administration
- Intravenous
- Intravitreal
- Oral
End-User
- Hospitals
- Specialty Clinics
- Research Institutions
- Pharmaceutical and Biopharmaceutical Companies
Global VEGF-B Inhibitors Market Regional Analysis
The market is analyzed and market size insights and trends are provided by country, drug class, therapeutic application, route of administration, and end-user as referenced above.
The countries covered in the market are U.S., Canada, Mexico, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, rest of Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, rest of Asia-Pacific, Saudi Arabia, U.A.E., South Africa, Egypt, Israel, rest of Middle East and Africa, Brazil, Argentina, and rest of South America.
North America is expected to dominate the market due to its well-established healthcare infrastructure, substantial investments in research and development, and the high demand for innovative, targeted therapies aimed at treating complex diseases. The region's strong presence of leading pharmaceutical and biotechnology companies further fosters the development and commercialization of cutting-edge treatments, ensuring its market leadership.
Asia-Pacific is expected to be the fastest growing due to increasing healthcare investments, a rising prevalence of chronic and autoimmune diseases, and the rapidly expanding biotechnology markets in key countries like China and India, which are enhancing access to advanced therapies and fueling market demand.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Global VEGF-B Inhibitors Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
Global VEGF-B Inhibitors Market Leaders Operating in the Market Are:
- Roche/Genentech (Switzerland/U.S.)
- Regeneron Pharmaceuticals (U.S.)
- Novartis AG (Switzerland)
- Pfizer Inc. (U.S.)
- Eli Lilly and Company (U.S.)
- Bayer AG (Germany)
- Amgen (U.S.)
- Biocon (India)
- Samsung Bioepis (South Korea)
- Alcon (Switzerland)
Latest Developments in Global VEGF-B Inhibitors Market
- In March 2021, Pfizer Inc. received FDA approval for a supplemental New Drug Application (sNDA) for LORBRENA (lorlatinib), expanding its indication to include first-line treatment for patients with anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer (NSCLC)
- On November 2023, the U.S. Food and Drug Administration (FDA) approved Augtyro™ (repotrectinib) for the treatment of adult patients with locally advanced or metastatic ROS1-positive non-small cell lung cancer (NSCLC)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.