Global Vitiligo Market
Market Size in USD Million
CAGR :
% 
 USD
1,127.12 Million
USD
1,837.54 Million
2021
2029
USD
1,127.12 Million
USD
1,837.54 Million
2021
2029
| 2022 –2029 | |
| USD 1,127.12 Million | |
| USD 1,837.54 Million | |
|
|
|
|
Market Analysis and Size
In recent years, the vitiligo market is anticipated to grow rapidly during the forecast period. Vitiligo is one of the 24 skin diseases listed by the American Academy of Dermatology Skin Disease. In 2013, more than 150,000 Americans of all ages were treated for vitiligo, at a cost of about USD 328 per patient, three times that of rosacea and double that of acne. Vitiligo is a skin disorder in which the pigmentation of the skin is lost in patches. Around the world, vitiligo affects 0.2 percent to 1% of the population. Vitiligo affects any part of the body when the skin's natural colour is lost due to a lack of melanin in the skin.
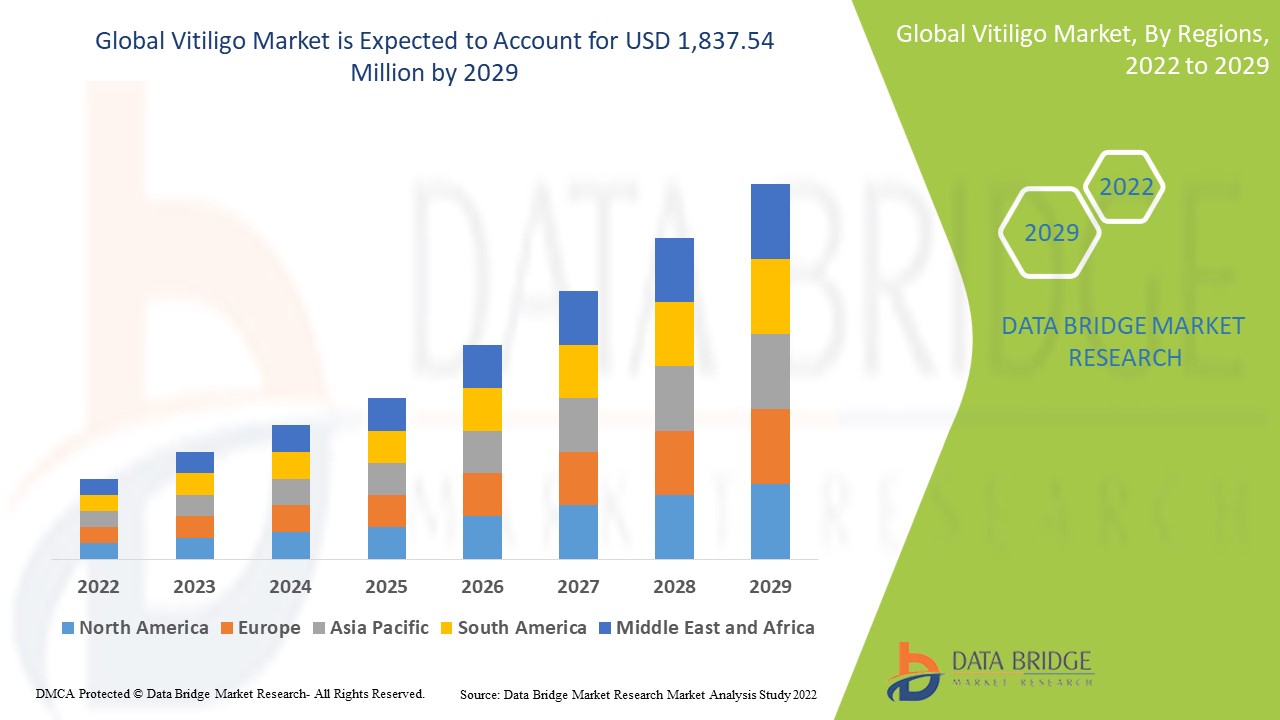
Data Bridge Market Research analyses that the Vitiligo market was valued at USD 1,127.12 million in 2021 and is expected to reach USD 1,837.54 million by 2029, registering a CAGR of 6.30% during the forecast period of 2022 to 2029. The market report curated by the Data Bridge Market Research team includes in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Market Definition
Vitiligo is a skin disorder in which colourless patches of skin develop. Vitiligo can affect the entire surface of the skin. This skin illness affects the eyes, the inside of the mouth, and the hair, and it differs from person to person. It's difficult to say how far the patches will expand and by how much. The emergence of white spots or patches on the skin is the most common symptom of vitiligo. Segmental vitiligo and non-segmental vitiligo are the two main kinds of vitiligo. Calcineurin inhibitors, corticosteroids, and psoralens are some of the most common medications prescribed by doctors to treat vitiligo.
Report Scope and Market Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2019 - 2014) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Type (Segmental Vitiligo, Non-Segmental Vitiligo, Others), Treatment (Medications, Therapies, Surgery, Others), Dosage Form (Oral, Injectable, Creams, Gel, Ointment, Others), Route of Administration (Oral, Parenteral, Topical, Others), End-Users (Hospitals, Specialty Clinics, Homecare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others) |
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Market Players Covered |
F. Hoffmann-La Roche Ltd. (Switzerland), Mylan N.V. (US), Teva Pharmaceutical Industries Ltd. (Israel), Sanofi (France), Pfizer Inc. (US), GlaxoSmithKline plc (UK), Novartis AG (Switzerland), Zydus Cadila (India), AstraZeneca (UK), Johnson & Johnson (US), Bayer AG (Germany), Sun Pharmaceutical Industries Ltd. (India), Merck & Co., Inc. (US), Bristol-Myers Squibb Company (US), Eli Lilly and Company (US), Cipla Inc. (US), LEO Pharma A/S (Denmark), Aurobindo Pharma (India), Lupin (India), Hikma Pharmaceuticals PLC (UK) |
|
Market Opportunities |
|
Vitiligo Market Dynamics
Drivers
- Increasing prevalence of vitiligo
The rising prevalence of vitiligo is estimated to enhance the market's growth. Its prevalence rate ranges between 0.2% and 4%. Vitiligo is a hypopigmentation skin condition in which the skin loses its colour due to a lack of melanin in the skin cells. It affects all portions of the body's skin and the hair and the inside of the mouth. Vitiligo is a skin condition that is neither life-threatening nor communicable. It affects everyone, regardless of their skin type.
- Increasing investment for healthcare infrastructure
Another significant factor influencing the growth rate of vitiligo market is the rising healthcare expenditure which helps in improving its infrastructure. Also, various government organizations aims to improve the healthcare infrastructure by increasing funding and this will further influence the market dynamics.
Furthermore, rising initiatives by public and private organizations to spread awareness will expand the Vitiligo market. Additionally, changing lifestyles of people and rising level of disposable income will result in the expansion of Vitiligo market. The growing importance of aesthetic appeal and increased adoption of cosmetic procedures and other dermatology procedures will propel the market's growth rate.
Opportunities
- Increase in the number of research and development activities
Moreover, the market's growth is fueled by an increase in the number of research and development activities. This will provide beneficial opportunities for the Vitiligo market growth. Along with this, rising drug approvals and launches will further propel the market's growth rate.
Moreover, rising investment for the development of advanced technologies and increase in the number of emerging markets will further provide beneficial opportunities for the Vitiligo market growth during the forecast period.
Restraints/Challenges
On the other hand, high cost associated with the treatment will obstruct the growth rate of market. The dearth of skilled professionals and lack of healthcare infrastructure in developing economies will challenge the Vitiligo market. Additionally, complications involved with vitiligo such as psychological distress, eye problems, sunburn and hearing loss, and lack of awareness among people will act as restrain and further impede the growth rate of market during the forecast period of 2022-2029.
This vitiligo market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the Vitiligo market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Patient Epidemiology Analysis
Vitiligo market also provides you with detailed market analysis for patient analysis, prognosis and cures. Prevalence, incidence, mortality, adherence rates are some of the data variables that are available in the report. Direct or indirect impact analyses of epidemiology to market growth are analysed to create a more robust and cohort multivariate statistical model for forecasting the market in the growth period.
COVID-19 Impact on Vitiligo Market
Since its emergence in December 2019, the COVID-19 virus has spread to nearly every country on the planet, prompting the World Health Organization (WHO) to declare it a public health emergency. COVID-19, a new coronavirus, was identified as the causal agent in pneumonia cases. This virus spread quickly over the world, killing a large number of people. COVID-19 was labelled a global pandemic by the World Health Organization (WHO) in March 2020, and rigorous measures to prevent the disease's spread were recommended. Since then, the pandemic has delayed the expansion of the healthcare sector and disrupted the supply chain. Furthermore, governments in many nations had imposed nationwide lockdowns to halt the spread of COVID-19. Similarly, healthcare organizations in numerous nations throughout the world were having difficulty continuing their supply chain activities. The supply chain slowness hampered the vitiligo market.
Recent Development
- In October 2021, Uniza Group had announced the launch of a novel solution named Vitellus for the management of vitiligo. Vitellus is a unique combination of Greyverse, Melitane, GL 200 & EUK-134 which is considered an advanced new age solution for managing vitiligo. It is lotion that helps in melamine growth and functions on skin pigmentation and hair follicle pigmentation.
Global Vitiligo Market Scope
The Vitiligo market is segmented on the basis of type, treatment, dosage form, route of administration, end-users and distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Type
- Segmental Vitiligo
- Non-Segmental Vitiligo
- Others
Treatment
- Medications
- Immunosuppressive
- Corticosteroids
Others
- Therapies
- Light Therapy
- Depigmentation
- Others
- Surgery
- Skin grafting
- Blister grafting
- Micropigmentation
- Others
- Others
Dosage Form
- Oral
- Injectable
- Creams
- Gel
- Ointment
- Others
Route of Administration
- Oral
- Parenteral
- Topical
- Others
End-Users
- Hospitals
- Specialty Clinics
- Homecare
- Others
Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
Vitiligo Market Regional Analysis/Insights
The vitiligo market is analysed and market size insights and trends are provided by country, type, treatment, dosage form, route of administration, end-users and distribution channel as referenced above.
The countries covered in the vitiligo market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America
North America dominates the vitiligo market because of the favourable reimbursement scenario and presence of major key players in this region. Additionally, rising healthcare expenditure will further propel the market's growth rate in this region.
Asia-Pacific is expected to grow during the forecast period due to the increasing number of cases of skin diseases due to sunlight exposure in this region. Also, development of healthcare infrastructure and rising awareness about the diseases will further propel the market's growth rate in this region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Vitiligo Market Share Analysis
The vitiligo market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to vitiligo market.
Some of the major players operating in the vitiligo market are:
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Mylan N.V. (US)
- Teva Pharmaceutical Industries Ltd.(Israel)
- Sanofi (France)
- Pfizer Inc. (US)
- GlaxoSmithKline plc (UK)
- Novartis AG (Switzerland)
- Zydus Cadila (India)
- AstraZeneca (UK)
- Johnson & Johnson (US)
- Bayer AG (Germany)
- Sun Pharmaceutical Industries Ltd. (India)
- Bristol-Myers Squibb Company (US)
- Eli Lilly and Company (US)
- Cipla Inc. (US)
- LEO Pharma A/S (Denmark)
- Aurobindo Pharma (India)
- Lupin (India)
- Hikma Pharmaceuticals PLC (UK)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF GLOBAL VITILIGO MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 KEY TAKEAWAYS
2.2 ARRIVING AT THE GLOBAL VITILIGO MARKET SIZE
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 TRIPOD DATA VALIDATION MODEL
2.2.4 MARKET GUIDE
2.2.5 MULTIVARIATE MODELLING
2.2.6 TOP TO BOTTOM ANALYSIS
2.2.7 CHALLENGE MATRIX
2.2.8 APPLICATION COVERAGE GRID
2.2.9 STANDARDS OF MEASUREMENT
2.2.10 VENDOR SHARE ANALYSIS
2.2.11 EPIDEMIOLOGY MODELING
2.2.12 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.13 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 GLOBAL VITILIGO MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMSUM INSIGHTS
4.1 PORTER'S 5 FORCES
4.2 PESTEL ANALYSIS
5 EPIDEMIOLOGY
5.1 INCIDENCE OF ALL BY GENDER
5.1.1 PREVALENCE OF VITILIGO
5.1.1.1. PREVALENCE E OF NONSEGMENTED VITILIGO
5.1.1.2. PREVALENCE OF SEGMENTED VITILIGO
5.1.1.3. PREVALENCE OF MIXED VITILIGO
5.1.2 INCEDENCE OF VITILIGO TREATED BY SURGERY
5.1.2.1. INCEDENCE OF VITILIGO TREATED BY SKIN GRAFT
5.1.2.2. INCEDENCE OF VITILIGO TREATED BY CELL TRANSPLANT
5.1.2.3. INCEDENCE OF VITILIGO TREATED BY MICROPIGMENTATION
5.2 TREATMENT RATE
5.3 MORTALITY RATE
5.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
5.5 PATEINT TREATMENT SUCCESS RATES
6 INDUSTRY INSIGHTS
6.1 PATENT ANALYSIS
6.2 DRUG TREATMENT RATE BY MATURED MARKETS
6.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
6.4 PATIENT FLOW DIAGRAM
6.5 KEY PRICING STRATEGIES
6.6 KEY PATIENT ENROLLMENT STRATEGIES
6.7 INTERVIEWS WITH PATHOLOGIST
6.8 OTHER KOL SNAPSHOTS
7 REGULATORY SCENARIO
7.1 FDA APPROVALS
7.2 EMA APPROVALS
8 MERGERS AND ACQUISITION
8.1 LICENSING
8.2 COMMERCIALIZATION AGREEMENTS
9 PIPELINE ANALYSIS
9.1 CLINICAL TRIALS AND PHASE ANALYSIS
9.2 DRUG THERAPY PIPELINE
9.3 PHASE III CANDIDATES
9.4 PHASE II CANDIDATES
9.4.1 RECELL DEVICE
9.4.2 SPRAY ON SKINTM CELLS
9.4.3 NB-UVB PHOTOTHERAPY
9.4.4 RAPAMYCIN
9.4.5 UPADACITINIB
9.4.6 INCB054707
9.4.7 AS012
9.4.8 AMG-714
9.4.9 TOFACITINIB
9.4.10 JAK1 INHIBITORS
9.4.10.1. PF-06651600
9.4.10.2. ARQ-252
9.4.10.3. CERDULATINIB
9.4.10.4. OTHERS
9.5 PHASE I CANDIDATE
9.5.1 VLRX001
9.5.2 BNZ-1
9.5.3 BOS-475
9.6 OTHERS (PRE-CLINICAL AND RESEARCH)
10 MARKET OVERVIEW
10.1 DRIVERS
10.2 RESTRAINS
10.3 OPPURTUNITY
10.4 CHALLENGES
11 GLOBAL VITILIGO MARKET, BY TYPE
11.1 OVERVIEW
11.2 NON SEGMENTAL VITILIGO
11.2.1 MEDICATION
11.2.1.1. TOPICAL CORTICOSTEROIDS
11.2.1.1.1. BY DRUGS
11.2.1.1.1.1 FLUTICASONE PROPIONATE
11.2.1.1.1.1.1. MARKET VALUE (USD MN)
11.2.1.1.1.1.2. MARKET VOLUME (SU)
11.2.1.1.1.1.3. AVERAGE SELLING PRICE (USD)
11.2.1.1.1.2 BETAMETHASONE VALERATE
11.2.1.1.1.2.1. MARKET VALUE (USD MN)
11.2.1.1.1.2.2. MARKET VOLUME (SU)
11.2.1.1.1.2.3. AVERAGE SELLING PRICE (USD)
11.2.1.1.1.3 HYDROCORTISONE BUTYRATE
11.2.1.1.1.3.1. MARKET VALUE (USD MN)
11.2.1.1.1.3.2. MARKET VOLUME (SU)
11.2.1.1.1.3.3. AVERAGE SELLING PRICE (USD)
11.2.1.1.1.4 OTHERS
11.2.1.1.2. BY DRUG TYPE
11.2.1.1.2.1 GENERICS
11.2.1.1.2.2 BRANDED
11.2.1.1.2.2.1. ARUNITY ELLIPTA
1.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
2.2.1.1.1.1.1.1 MARKET VOLUME (SU)
3.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.2.1.1.2.2.2. FLOVENT
4.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
5.2.1.1.1.1.1.1 MARKET VOLUME (SU)
6.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.2.1.1.2.2.3. CELESTONE
7.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
8.2.1.1.1.1.1.1 MARKET VOLUME (SU)
9.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.2.1.1.2.2.4. HYDROCORT
10.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
11.2.1.1.1.1.1.1 MARKET VOLUME (SU)
12.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.2.1.1.2.2.5. ALPHOSYL
13.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
14.2.1.1.1.1.1.1 MARKET VOLUME (SU)
15.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.2.1.1.2.2.6. OTHERS
11.2.1.2. CALCINEURIN INHIBITORS
11.2.1.2.1. BY DRUGS
11.2.1.2.1.1 TACROLIMUS
11.2.1.2.1.1.1. MARKET VALUE (USD MN)
11.2.1.2.1.1.2. MARKET VOLUME (SU)
11.2.1.2.1.1.3. AVERAGE SELLING PRICE (USD)
11.2.1.2.1.2 PIMECROLIMUS
11.2.1.2.1.2.1. MARKET VALUE (USD MN)
11.2.1.2.1.2.2. MARKET VOLUME (SU)
11.2.1.2.1.2.3. AVERAGE SELLING PRICE (USD)
11.2.1.2.1.3 OTHERS
11.2.1.2.2. BY DRUG TYPE
11.2.1.2.2.1 GENERICS
11.2.1.2.2.2 BRANDED
11.2.1.2.2.2.1. ASTAGRAF XL
16.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
17.2.1.1.1.1.1.1 MARKET VOLUME (SU)
18.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.2.1.2.2.2.2. ENVARSUSXR
19.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
20.2.1.1.1.1.1.1 MARKET VOLUME (SU)
21.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.2.1.2.2.2.3. PROGRAF
22.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
23.2.1.1.1.1.1.1 MARKET VOLUME (SU)
24.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.2.1.2.2.2.4. ELIDED
25.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
26.2.1.1.1.1.1.1 MARKET VOLUME (SU)
27.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.2.1.2.2.2.5. OTHERS
11.2.1.3. CALCIPOTRIENE (DOVONEX)
11.2.1.3.1. MARKET VALUE (USD MN)
11.2.1.3.2. MARKET VOLUME (SU)
11.2.1.3.3. AVERAGE SELLING PRICE (USD)
11.2.1.4. RUXOLITINIB (OPZELURA)
11.2.1.4.1. MARKET VALUE (USD MN)
11.2.1.4.2. MARKET VOLUME (SU)
11.2.1.4.3. AVERAGE SELLING PRICE (USD)
11.2.1.5. VITAMIN D
11.2.1.5.1. MARKET VALUE (USD MN)
11.2.1.5.2. MARKET VOLUME (SU)
11.2.1.5.3. AVERAGE SELLING PRICE (USD)
11.2.1.6. OTHERS
11.2.2 THERAPY
11.2.2.1. LIGHT THERAPY
11.2.2.2. REPIGMENTATION THERAPY
11.2.2.3. CAMOUFLAGE THERAPY
11.2.3 SURGERY
11.2.3.1. SKIN GRAFT
11.2.3.2. CELL TRANSPLANT
11.2.3.3. MICROPIGMENTATION
11.2.3.4. OTHERS
11.3 SEGMENTAL VITILIGO
11.3.1 MEDICATION
11.3.1.1. TOPICAL CORTICOSTEROIDS
11.3.1.1.1. BY DRUGS
11.3.1.1.1.1 FLUTICASONE PROPIONATE
11.3.1.1.1.1.1. MARKET VALUE (USD MN)
11.3.1.1.1.1.2. MARKET VOLUME (SU)
11.3.1.1.1.1.3. AVERAGE SELLING PRICE (USD)
11.3.1.1.1.2 BETAMETHASONE VALERATE
11.3.1.1.1.2.1. MARKET VALUE (USD MN)
11.3.1.1.1.2.2. MARKET VOLUME (SU)
11.3.1.1.1.2.3. AVERAGE SELLING PRICE (USD)
11.3.1.1.1.3 HYDROCORTISONE BUTYRATE
11.3.1.1.1.3.1. MARKET VALUE (USD MN)
11.3.1.1.1.3.2. MARKET VOLUME (SU)
11.3.1.1.1.3.3. AVERAGE SELLING PRICE (USD)
11.3.1.1.1.4 OTHERS
11.3.1.1.2. BY DRUG TYPE
11.3.1.1.2.1 GENERICS
11.3.1.1.2.2 BRANDED
11.3.1.1.2.2.1. ARUNITY ELLIPTA
28.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
29.2.1.1.1.1.1.1 MARKET VOLUME (SU)
30.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.3.1.1.2.2.2. FLOVENT
31.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
32.2.1.1.1.1.1.1 MARKET VOLUME (SU)
33.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.3.1.1.2.2.3. CELESTONE
34.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
35.2.1.1.1.1.1.1 MARKET VOLUME (SU)
36.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.3.1.1.2.2.4. HYDROCORT
37.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
38.2.1.1.1.1.1.1 MARKET VOLUME (SU)
39.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.3.1.1.2.2.5. ALPHOSYL
40.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
41.2.1.1.1.1.1.1 MARKET VOLUME (SU)
42.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.3.1.1.2.2.6. OTHERS
11.3.1.2. CALCINEURIN INHIBITORS
11.3.1.2.1. BY DRUGS
11.3.1.2.1.1 TACROLIMUS
11.3.1.2.1.1.1. MARKET VALUE (USD MN)
11.3.1.2.1.1.2. MARKET VOLUME (SU)
11.3.1.2.1.1.3. AVERAGE SELLING PRICE (USD)
11.3.1.2.1.2 PIMECROLIMUS
11.3.1.2.1.2.1. MARKET VALUE (USD MN)
11.3.1.2.1.2.2. MARKET VOLUME (SU)
11.3.1.2.1.2.3. AVERAGE SELLING PRICE (USD)
11.3.1.2.1.3 OTHERS
11.3.1.2.2. BY DRUG TYPE
11.3.1.2.2.1 GENERICS
11.3.1.2.2.2 BRANDED
11.3.1.2.2.2.1. ASTAGRAF XL
43.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
44.2.1.1.1.1.1.1 MARKET VOLUME (SU)
45.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.3.1.2.2.2.2. ENVARSUSXR
46.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
47.2.1.1.1.1.1.1 MARKET VOLUME (SU)
48.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.3.1.2.2.2.3. PROGRAF
49.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
50.2.1.1.1.1.1.1 MARKET VOLUME (SU)
51.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.3.1.2.2.2.4. ELIDED
52.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
53.2.1.1.1.1.1.1 MARKET VOLUME (SU)
54.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.3.1.2.2.2.5. OTHERS
11.3.1.3. CALCIPOTRIENE (DOVONEX)
11.3.1.3.1. MARKET VALUE (USD MN)
11.3.1.3.2. MARKET VOLUME (SU)
11.3.1.3.3. AVERAGE SELLING PRICE (USD)
11.3.1.4. RUXOLITINIB (OPZELURA)
11.3.1.4.1. MARKET VALUE (USD MN)
11.3.1.4.2. MARKET VOLUME (SU)
11.3.1.4.3. AVERAGE SELLING PRICE (USD)
11.3.1.5. VITAMIN D
11.3.1.5.1. MARKET VALUE (USD MN)
11.3.1.5.2. MARKET VOLUME (SU)
11.3.1.5.3. AVERAGE SELLING PRICE (USD)
11.3.1.6. OTHERS
11.3.2 THERAPY
11.3.2.1. LIGHT THERAPY
11.3.2.2. REPIGMENTATION THERAPY
11.3.2.3. CAMOUFLAGE THERAPY
11.3.3 SURGERY
11.3.3.1. SKIN GRAFT
11.3.3.2. CELL TRANSPLANT
11.3.3.3. MICROPIGMENTATION
11.3.3.4. OTHERS
11.4 MIXED VITILIGO
11.4.1 MEDICATION
11.4.1.1. TOPICAL CORTICOSTEROIDS
11.4.1.1.1. BY DRUGS
11.4.1.1.1.1 FLUTICASONE PROPIONATE
11.4.1.1.1.1.1. MARKET VALUE (USD MN)
11.4.1.1.1.1.2. MARKET VOLUME (SU)
11.4.1.1.1.1.3. AVERAGE SELLING PRICE (USD)
11.4.1.1.1.2 BETAMETHASONE VALERATE
11.4.1.1.1.2.1. MARKET VALUE (USD MN)
11.4.1.1.1.2.2. MARKET VOLUME (SU)
11.4.1.1.1.2.3. AVERAGE SELLING PRICE (USD)
11.4.1.1.1.3 HYDROCORTISONE BUTYRATE
11.4.1.1.1.3.1. MARKET VALUE (USD MN)
11.4.1.1.1.3.2. MARKET VOLUME (SU)
11.4.1.1.1.3.3. AVERAGE SELLING PRICE (USD)
11.4.1.1.1.4 OTHERS
11.4.1.1.2. BY DRUG TYPE
11.4.1.1.2.1 GENERICS
11.4.1.1.2.2 BRANDED
11.4.1.1.2.2.1. ARUNITY ELLIPTA
55.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
56.2.1.1.1.1.1.1 MARKET VOLUME (SU)
57.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.4.1.1.2.2.2. FLOVENT
58.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
59.2.1.1.1.1.1.1 MARKET VOLUME (SU)
60.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.4.1.1.2.2.3. CELESTONE
61.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
62.2.1.1.1.1.1.1 MARKET VOLUME (SU)
63.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.4.1.1.2.2.4. HYDROCORT
64.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
65.2.1.1.1.1.1.1 MARKET VOLUME (SU)
66.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.4.1.1.2.2.5. ALPHOSYL
67.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
68.2.1.1.1.1.1.1 MARKET VOLUME (SU)
69.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.4.1.1.2.2.6. OTHERS
11.4.1.2. CALCINEURIN INHIBITORS
11.4.1.2.1. BY DRUGS
11.4.1.2.1.1 TACROLIMUS
11.4.1.2.1.1.1. MARKET VALUE (USD MN)
11.4.1.2.1.1.2. MARKET VOLUME (SU)
11.4.1.2.1.1.3. AVERAGE SELLING PRICE (USD)
11.4.1.2.1.2 PIMECROLIMUS
11.4.1.2.1.2.1. MARKET VALUE (USD MN)
11.4.1.2.1.2.2. MARKET VOLUME (SU)
11.4.1.2.1.2.3. AVERAGE SELLING PRICE (USD)
11.4.1.2.1.3 OTHERS
11.4.1.2.2. BY DRUG TYPE
11.4.1.2.2.1 GENERICS
11.4.1.2.2.2 BRANDED
11.4.1.2.2.2.1. ASTAGRAF XL
70.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
71.2.1.1.1.1.1.1 MARKET VOLUME (SU)
72.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.4.1.2.2.2.2. ENVARSUSXR
73.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
74.2.1.1.1.1.1.1 MARKET VOLUME (SU)
75.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.4.1.2.2.2.3. PROGRAF
76.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
77.2.1.1.1.1.1.1 MARKET VOLUME (SU)
78.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.4.1.2.2.2.4. ELIDED
79.2.1.1.1.1.1.1 MARKET VALUE (USD MN)
80.2.1.1.1.1.1.1 MARKET VOLUME (SU)
81.2.1.1.1.1.1.1 AVERAGE SELLING PRICE (USD)
11.4.1.2.2.2.5. OTHERS
11.4.1.3. CALCIPOTRIENE (DOVONEX)
11.4.1.3.1. MARKET VALUE (USD MN)
11.4.1.3.2. MARKET VOLUME (SU)
11.4.1.3.3. AVERAGE SELLING PRICE (USD)
11.4.1.4. RUXOLITINIB (OPZELURA)
11.4.1.4.1. MARKET VALUE (USD MN)
11.4.1.4.2. MARKET VOLUME (SU)
11.4.1.4.3. AVERAGE SELLING PRICE (USD)
11.4.1.5. VITAMIN D
11.4.1.5.1. MARKET VALUE (USD MN)
11.4.1.5.2. MARKET VOLUME (SU)
11.4.1.5.3. AVERAGE SELLING PRICE (USD)
11.4.1.6. OTHERS
11.4.2 THERAPY
11.4.2.1. LIGHT THERAPY
11.4.2.2. REPIGMENTATION THERAPY
11.4.2.3. CAMOUFLAGE THERAPY
11.4.3 SURGERY
11.4.3.1. SKIN GRAFT
11.4.3.2. CELL TRANSPLANT
11.4.3.3. MICROPIGMENTATION
11.4.3.4. OTHERS
11.5 OTHERS VITILIGO
12 GLOBAL VITILIGO MARKET, BY ROUTE OF ADMINISTRATION
12.1 OVERVIEW
12.2 TOPICAL
12.3 OTHERS
13 GLOBAL VITILIGO MARKET, BY END USER
13.1 OVERVIEW
13.2 HOSPITALS
13.3 DERMATOLOGY CLINICS
13.4 ACADEMIC AND RESEARCH INSTITUTES
13.5 OTHERS
14 GLOBAL VITILIGO MARKET, BY DISTRIBUTION CHANNEL
14.1 OVERVIEW
14.2 HOSPITAL PHARMACY
14.3 RETAIL PHARMACY
14.4 OTHERS
15 GLOBAL VITILIGO MARKET, BY GEOGRAPHY
15.1 GLOBAL VITILIGO MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
15.2 NORTH AMERICA
15.2.1 U.S.
15.2.1.1. U.S. VITILIGO MARKET, BY TYPE
15.2.1.2. U.S. VITILIGO MARKET, BY ROUTE OF ADMINISTRATION
15.2.1.3. U.S. VITILIGO MARKET, BY END USER
15.2.1.4. U.S. VITILIGO MARKET, BY DISTRIBUTION CHANNEL
15.2.2 CANADA
15.2.3 MEXICO
15.2.4 DOMINICAN REPUBLIC
15.2.5 JAMAICA
15.2.6 PANAMA
15.3 EUROPE
15.3.1 GERMANY
15.3.2 FRANCE
15.3.3 U.K.
15.3.4 HUNGARY
15.3.5 LITHUANIA
15.3.6 AUSTRIA
15.3.7 IRELAND
15.3.8 NORWAY
15.3.9 POLAND
15.3.10 ITALY
15.3.11 SPAIN
15.3.12 RUSSIA
15.3.13 TURKEY
15.3.14 NETHERLANDS
15.3.15 SWITZERLAND
15.3.16 REST OF EUROPE
15.4 ASIA-PACIFIC
15.4.1 JAPAN
15.4.2 CHINA
15.4.3 TAIWAN
15.4.4 SOUTH KOREA
15.4.5 INDIA
15.4.6 AUSTRALIA
15.4.7 SINGAPORE
15.4.8 THAILAND
15.4.9 MALAYSIA
15.4.10 INDONESIA
15.4.11 PHILIPPINES
15.4.12 VIETNAM
15.4.13 REST OF ASIA-PACIFIC
15.5 SOUTH AMERICA
15.5.1 BRAZIL
15.5.2 ECUADOR
15.5.3 CHILE
15.5.4 COLOMBIA
15.5.5 VENEZUELA
15.5.6 ARGENTINA
15.5.7 PERU
15.5.8 CURAÇAO
15.5.9 PARAGUAY
15.5.10 URUGUAY
15.5.11 TRINIDAD AND TOBAGO
15.5.12 REST OF SOUTH AMERICA
15.6 MIDDLE EAST AND AFRICA
15.6.1 SOUTH AFRICA
15.6.2 SAUDI ARABIA
15.6.3 UAE
15.6.4 EGYPT
15.6.5 KUWAIT
15.6.6 ISRAEL
15.6.7 BOLIVIA
15.6.8 REST OF MIDDLE EAST AND AFRICA
15.7 KEY PRIMARY INSIGHTS: BY MAJOR COUNTRIES
16 GLOBAL VITILIGO MARKET, SWOT AND DBMR ANALYSIS
17 GLOBAL VITILIGO MARKET, COMPANY LANDSCAPE
17.1 COMPANY SHARE ANALYSIS: GLOBAL
17.2 COMPANY SHARE ANALYSIS: NORTH AMERICA
17.3 COMPANY SHARE ANALYSIS: EUROPE
17.4 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
17.5 MERGERS & ACQUISITIONS
17.6 NEW PRODUCT DEVELOPMENT & APPROVALS
17.7 EXPANSIONS
17.8 REGULATORY CHANGES
17.9 PARTNERSHIP AND OTHER STRATEGIC DEVELOPMENTS
18 GLOBAL VITILIGO MARKET, COMPANY PROFILE
18.1 MAUFACTURING COMPANIES
18.1.1 INCYTE CORPORATION
18.1.1.1. COMPANY OVERVIEW
18.1.1.2. REVENUE ANALYSIS
18.1.1.3. GEOGRAPHIC PRESENCE
18.1.1.4. PRODUCT PORTFOLIO
18.1.1.5. RECENT DEVELOPMENTS
18.1.2 GLENMARK PHARMACEUTICALS
18.1.2.1. COMPANY OVERVIEW
18.1.2.2. REVENUE ANALYSIS
18.1.2.3. GEOGRAPHIC PRESENCE
18.1.2.4. PRODUCT PORTFOLIO
18.1.2.5. RECENT DEVELOPMENTS
18.1.3 GSK
18.1.3.1. COMPANY OVERVIEW
18.1.3.2. REVENUE ANALYSIS
18.1.3.3. GEOGRAPHIC PRESENCE
18.1.3.4. PRODUCT PORTFOLIO
18.1.3.5. RECENT DEVELOPMENTS
18.1.4 BAUSCH & LOMB
18.1.4.1. COMPANY OVERVIEW
18.1.4.2. REVENUE ANALYSIS
18.1.4.3. GEOGRAPHIC PRESENCE
18.1.4.4. PRODUCT PORTFOLIO
18.1.4.5. RECENT DEVELOPMENTS
18.1.5 DR REDDY LABORATORIES
18.1.5.1. COMPANY OVERVIEW
18.1.5.2. REVENUE ANALYSIS
18.1.5.3. GEOGRAPHIC PRESENCE
18.1.5.4. PRODUCT PORTFOLIO
18.1.5.5. RECENT DEVELOPMENTS
18.1.6 ACCORD HEALTHCARE
18.1.6.1. COMPANY OVERVIEW
18.1.6.2. REVENUE ANALYSIS
18.1.6.3. GEOGRAPHIC PRESENCE
18.1.6.4. PRODUCT PORTFOLIO
18.1.6.5. RECENT DEVELOPMENTS
18.1.7 PANACEA BIOTECH
18.1.7.1. COMPANY OVERVIEW
18.1.7.2. REVENUE ANALYSIS
18.1.7.3. GEOGRAPHIC PRESENCE
18.1.7.4. PRODUCT PORTFOLIO
18.1.7.5. RECENT DEVELOPMENTS
18.1.8 SANDOZ INTERNATIONAL GMBH
18.1.8.1. COMPANY OVERVIEW
18.1.8.2. REVENUE ANALYSIS
18.1.8.3. GEOGRAPHIC PRESENCE
18.1.8.4. PRODUCT PORTFOLIO
18.1.8.5. RECENT DEVELOPMENTS
18.1.9 VIATRIS (MYLAN)
18.1.9.1. COMPANY OVERVIEW
18.1.9.2. REVENUE ANALYSIS
18.1.9.3. GEOGRAPHIC PRESENCE
18.1.9.4. PRODUCT PORTFOLIO
18.1.9.5. RECENT DEVELOPMENTS
18.1.10 NOVARTIS
18.1.10.1. COMPANY OVERVIEW
18.1.10.2. REVENUE ANALYSIS
18.1.10.3. GEOGRAPHIC PRESENCE
18.1.10.4. PRODUCT PORTFOLIO
18.1.10.5. RECENT DEVELOPMENTS
18.1.11 GALDERMA
18.1.11.1. COMPANY OVERVIEW
18.1.11.2. REVENUE ANALYSIS
18.1.11.3. GEOGRAPHIC PRESENCE
18.1.11.4. PRODUCT PORTFOLIO
18.1.11.5. RECENT DEVELOPMENTS
18.1.12 TEVA PHARMACEUTICALS
18.1.12.1. COMPANY OVERVIEW
18.1.12.2. REVENUE ANALYSIS
18.1.12.3. GEOGRAPHIC PRESENCE
18.1.12.4. PRODUCT PORTFOLIO
18.1.12.5. RECENT DEVELOPMENTS
18.1.13 CIPLA, INC
18.1.13.1. COMPANY OVERVIEW
18.1.13.2. REVENUE ANALYSIS
18.1.13.3. GEOGRAPHIC PRESENCE
18.1.13.4. PRODUCT PORTFOLIO
18.1.13.5. RECENT DEVELOPMENTS
18.1.14 RALINGTON PHARMA
18.1.14.1. COMPANY OVERVIEW
18.1.14.2. REVENUE ANALYSIS
18.1.14.3. GEOGRAPHIC PRESENCE
18.1.14.4. PRODUCT PORTFOLIO
18.1.14.5. RECENT DEVELOPMENTS
18.1.15 JOHNSON & JOHNSON
18.1.15.1. COMPANY OVERVIEW
18.1.15.2. REVENUE ANALYSIS
18.1.15.3. GEOGRAPHIC PRESENCE
18.1.15.4. PRODUCT PORTFOLIO
18.1.15.5. RECENT DEVELOPMENTS
18.1.16 ELI LILLY
18.1.16.1. COMPANY OVERVIEW
18.1.16.2. REVENUE ANALYSIS
18.1.16.3. GEOGRAPHIC PRESENCE
18.1.16.4. PRODUCT PORTFOLIO
18.1.16.5. RECENT DEVELOPMENTS
18.1.17 SANOFI
18.1.17.1. COMPANY OVERVIEW
18.1.17.2. REVENUE ANALYSIS
18.1.17.3. GEOGRAPHIC PRESENCE
18.1.17.4. PRODUCT PORTFOLIO
18.1.17.5. RECENT DEVELOPMENTS
18.1.18 BOEHRINGER INGELHEIM
18.1.18.1. COMPANY OVERVIEW
18.1.18.2. REVENUE ANALYSIS
18.1.18.3. GEOGRAPHIC PRESENCE
18.1.18.4. PRODUCT PORTFOLIO
18.1.18.5. RECENT DEVELOPMENTS
18.1.19 ASTELLAS PHARMA
18.1.19.1. COMPANY OVERVIEW
18.1.19.2. REVENUE ANALYSIS
18.1.19.3. GEOGRAPHIC PRESENCE
18.1.19.4. PRODUCT PORTFOLIO
18.1.19.5. RECENT DEVELOPMENTS
18.1.20 BAXTER INTERNATIONAL INC
18.1.20.1. COMPANY OVERVIEW
18.1.20.2. REVENUE ANALYSIS
18.1.20.3. GEOGRAPHIC PRESENCE
18.1.20.4. PRODUCT PORTFOLIO
18.1.20.5. RECENT DEVELOPMENTS
18.1.21 PFIZER
18.1.21.1. COMPANY OVERVIEW
18.1.21.2. REVENUE ANALYSIS
18.1.21.3. GEOGRAPHIC PRESENCE
18.1.21.4. PRODUCT PORTFOLIO
18.1.21.5. RECENT DEVELOPMENTS
18.2 PIPELINE COMPANIES
18.2.1 AVITA MEDICAL
18.2.1.1. COMPANY OVERVIEW
18.2.1.2. REVENUE ANALYSIS
18.2.1.3. GEOGRAPHIC PRESENCE
18.2.1.4. PRODUCT PORTFOLIO
18.2.1.5. RECENT DEVELOPMENTS
18.2.2 ARCUTIS BIOTHERAPEUTICS
18.2.2.1. COMPANY OVERVIEW
18.2.2.2. REVENUE ANALYSIS
18.2.2.3. GEOGRAPHIC PRESENCE
18.2.2.4. PRODUCT PORTFOLIO
18.2.2.5. RECENT DEVELOPMENTS
18.2.3 CLINUVEL PHARMACEUTICALS
18.2.3.1. COMPANY OVERVIEW
18.2.3.2. REVENUE ANALYSIS
18.2.3.3. GEOGRAPHIC PRESENCE
18.2.3.4. PRODUCT PORTFOLIO
18.2.3.5. RECENT DEVELOPMENTS
18.2.4 EQUILLSUM BIO (BIONIZ THERAPEUTICS)
18.2.4.1. COMPANY OVERVIEW
18.2.4.2. REVENUE ANALYSIS
18.2.4.3. GEOGRAPHIC PRESENCE
18.2.4.4. PRODUCT PORTFOLIO
18.2.4.5. RECENT DEVELOPMENTS
18.2.5 BOSTON PHARMACEUTICALS
18.2.5.1. COMPANY OVERVIEW
18.2.5.2. REVENUE ANALYSIS
18.2.5.3. GEOGRAPHIC PRESENCE
18.2.5.4. PRODUCT PORTFOLIO
18.2.5.5. RECENT DEVELOPMENTS
18.2.6 ARCUTIS BIOTHERAPEUTICS
18.2.6.1. COMPANY OVERVIEW
18.2.6.2. REVENUE ANALYSIS
18.2.6.3. GEOGRAPHIC PRESENCE
18.2.6.4. PRODUCT PORTFOLIO
18.2.6.5. RECENT DEVELOPMENTS
18.2.7 ROVIANT SCIENCES
18.2.7.1. COMPANY OVERVIEW
18.2.7.2. REVENUE ANALYSIS
18.2.7.3. GEOGRAPHIC PRESENCE
18.2.7.4. PRODUCT PORTFOLIO
18.2.7.5. RECENT DEVELOPMENTS
18.2.8 DERMAVANT SCIENCES
18.2.8.1. COMPANY OVERVIEW
18.2.8.2. REVENUE ANALYSIS
18.2.8.3. GEOGRAPHIC PRESENCE
18.2.8.4. PRODUCT PORTFOLIO
18.2.8.5. RECENT DEVELOPMENTS
NOTE: THE COMPANIES PROFILED IS NOT EXHAUSTIVE LIST AND IS AS PER OUR PREVIOUS CLIENT REQUIREMENT. WE PROFILE MORE THAN 100 COMPANIES IN OUR STUDY AND HENCE THE LIST OF COMPANIES CAN BE MODIFIED OR REPLACED ON REQUEST
19 RELATED REPORTS
20 CONCLUSION
21 QUESTIONNAIRE
22 ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.