Global X Linked Adrenoleukodystrophy Market
Market Size in USD Billion
CAGR :
% 
 USD
1.70 Billion
USD
2.94 Billion
2024
2032
USD
1.70 Billion
USD
2.94 Billion
2024
2032
| 2025 –2032 | |
| USD 1.70 Billion | |
| USD 2.94 Billion | |
|
|
|
|
X-linked Adrenoleukodystrophy Market Size
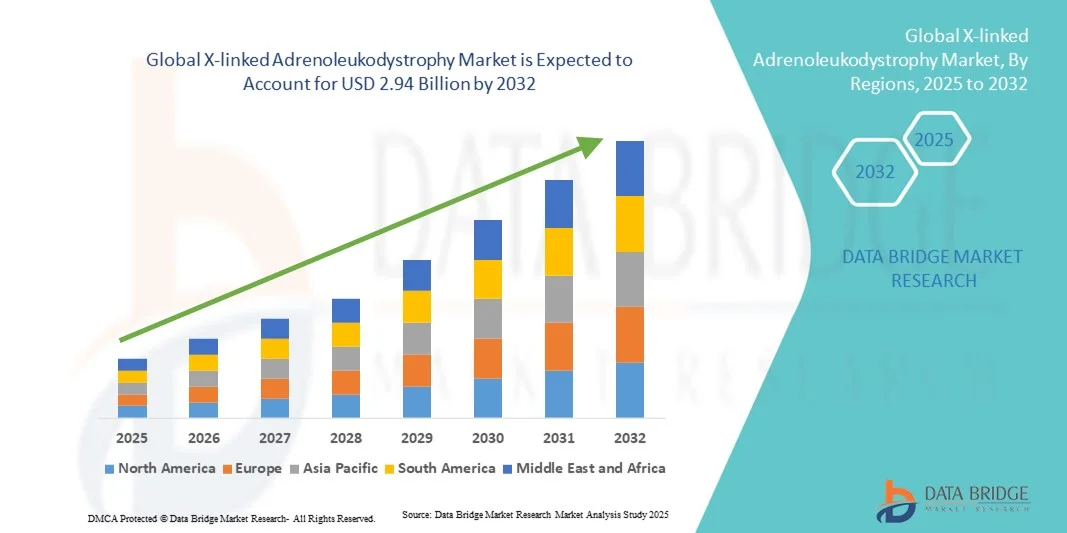
- The global X-linked adrenoleukodystrophy market size was valued at USD 1.70 billion in 2024 and is expected to reach USD 2.94 billion by 2032, at a CAGR of 7.10% during the forecast period
- The market growth is largely fueled by the increasing prevalence of X-linked Adrenoleukodystrophy (X-ALD) worldwide and the rising awareness of rare genetic disorders, leading to higher diagnosis rates and demand for advanced therapeutic solutions. Growing research in gene therapy, enzyme replacement, and stem cell-based treatments has significantly contributed to improving patient outcomes, thereby driving substantial progress in the overall X-ALD market
- Furthermore, the introduction of novel therapeutics and expanding clinical trials aimed at addressing the underlying genetic mutations of X-ALD are creating new avenues for market growth. The increasing collaboration between pharmaceutical companies, academic institutions, and research organizations is accelerating innovation in treatment modalities. These converging factors are fueling the uptake of X-linked Adrenoleukodystrophy solutions, thereby significantly boosting the industry's growth
X-linked Adrenoleukodystrophy Market Analysis
- X-linked Adrenoleukodystrophy (X-ALD), a rare genetic disorder affecting the nervous system and adrenal glands, is witnessing growing attention globally due to increasing diagnosis rates, advancements in genetic testing, and heightened awareness of inherited metabolic diseases. The market is being fueled by a surge in clinical research on gene therapy, enzyme replacement therapy, and stem cell transplantation, offering improved survival and quality of life for patients
- The accelerating demand for effective X-ALD treatments is primarily driven by expanding newborn screening programs, government initiatives supporting rare disease management, and continuous technological innovations in molecular diagnostics and gene editing. Increasing collaboration among pharmaceutical companies and research organizations is further propelling therapeutic development and accessibility
- North America dominated the X-linked adrenoleukodystrophy market with the largest revenue share of 41.6% in 2024, owing to strong healthcare infrastructure, favorable regulatory support for orphan drugs, and the presence of key players such as Bluebird Bio and Minoryx Therapeutics. The U.S. leads the regional growth, driven by early adoption of gene therapy, rising clinical trial activities, and improved reimbursement policies for rare disease treatments
- Asia-Pacific is projected to be the fastest-growing region in the X-linked adrenoleukodystrophy market during the forecast period (2025–2032), exhibiting a CAGR of 8.9%, driven by growing healthcare expenditure, expanding access to genetic diagnostics, and increasing awareness campaigns in countries such as China, Japan, and India
- The MRI segment dominated the largest market revenue share of 45.7% in 2024, because neuroimaging is central to detecting cerebral demyelination, tracking disease progression, and guiding treatment decisions such as HSCT
Report Scope and X-linked Adrenoleukodystrophy Market Segmentation
|
Attributes |
X-linked Adrenoleukodystrophy Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
North America
Europe
Asia-Pacific
Middle East and Africa
South America
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
X-linked Adrenoleukodystrophy Market Trends
Enhanced Diagnostic Accessibility and Advanced Therapeutic Integration
- A significant and accelerating trend in the global X-ALD market is the deepening integration of advanced diagnostics, gene-therapy platforms, and multidisciplinary care models. This fusion of diagnostic and therapeutic technologies is significantly enhancing early disease detection, precision treatment strategies, and patient-management outcomes
- For instance, expanded newborn-screening programmes and next-generation sequencing panels are enabling earlier identification of X-ALD (including childhood cerebral ALD and adrenomyeloneuropathy). These systems allow clinicians to initiate treatment sooner and monitor progression with greater accuracy
- The integration of gene therapies, hematopoietic stem cell transplantation (HSCT), VLCFA-lowering agents, and multidisciplinary neurology-endocrinology care enables features such as personalised risk stratification, tailored therapeutic regimens, and improved long-term monitoring. Furthermore, treatment models that combine neurologic rehabilitation and endocrine support offer enhanced patient convenience and clinical efficiency
- This trend towards more integrated, patient-centric, and technologically advanced care pathways is fundamentally reshaping expectations for X-ALD management. Consequently, companies and research networks are developing comprehensive platforms combining early diagnostics, gene-therapy access, long-term monitoring, and coordinated care across neurology, endocrinology, and transplantation specialties
- The demand for solutions that offer seamless integration of diagnostics, novel therapeutics, and follow-up care is growing rapidly across both pediatric and adult patient populations, as healthcare providers increasingly prioritise early intervention, functional preservation and improved quality of life
X-linked Adrenoleukodystrophy Market Dynamics
Driver
Growing Need Due to Rising Awareness of Rare Neurologic-Endocrine Disorders and Therapeutic Innovation
- The increasing recognition and diagnosis of X-ALD — a rare, hereditary disorder affecting the adrenal glands and central nervous system — coupled with rapid advances in gene therapy, stem-cell transplantation and VLCFA-targeting treatments, is a major growth driver for the X-ALD market
- For instance, the global X-ALD drugs market was valued at around USD 519.45 million in 2024, and is projected to reach approximately USD 1,915.60 million by 2034, reflecting a compound annual growth rate (CAGR) of about 13.94% between 2025 and 2034
- As healthcare systems place greater emphasis on newborn screening, rare-disease registries and precision medicine pathways, the uptake of advanced diagnostics and novel therapies for X-ALD is increasing significantly
- Furthermore, regulatory incentives for orphan drug development, increased R&D investment in gene therapies and expanding access to transplantation and supportive care are driving market growth
- The increasing availability of diagnostics and therapeutic platforms — combined with the imperative for early treatment to improve outcomes — are key factors propelling adoption of X-ALD solutions across major regions
Restraint/Challenge
Limited Patient Numbers, High Treatment Costs and Complex Care Pathways
- The rarity of X-ALD and the highly specialised nature of its management remain key barriers to growth. Because X-ALD affects roughly 1 in 17,000 births, large-scale clinical studies and broad-market access for treatments are inherently constrained
- For instance, treatments like HSCT, gene therapy and lifelong monitoring require complex infrastructure, multidisciplinary teams and high cost — which limit access in lower-resource regions
- In addition, while gene therapies and novel modalities are promising, the long-term efficacy, safety and cost-effectiveness profiles are still being established — thereby slowing broader market adoption
- Furthermore, patient management demands coordination across neurology, endocrinology, transplantation and rehabilitation services — which adds to logistical burden and may restrict uptake in fragmented health-system
- Overcoming these challenges will require improved global awareness programmes, expanded rare-disease networks, reimbursement models for high-cost therapies and broader infrastructure for advanced treatments
X-linked Adrenoleukodystrophy Market Scope
The market is segmented on the basis of type, symptoms, treatment, diagnosis, end-users, and distribution channel.
- By Type
On the basis of type, the X-linked Adrenoleukodystrophy (ALD) market is segmented into Adrenomyeloneuropathy (AMN), Adult cerebral ALD, Childhood cerebral ALD, and Addison’s-only ALD. The Childhood cerebral ALD segment dominated the largest market revenue share of 41.2% in 2024, driven by its aggressive course, high clinical visibility, and substantial demand for intensive interventions. Childhood cerebral ALD typically presents with rapid neurological decline, prompting urgent diagnostic workups and therapeutic action that generate significant clinical and economic activity. The severity of presentations leads to higher hospitalization, imaging, and treatment utilization compared with milder phenotypes. Screening programs and newborn/adolescent surveillance in high-risk families have increased case detection, concentrating market value in this subtype. Specialized care pathways, including hematopoietic stem cell transplantation for early cerebral disease, further elevate resource consumption in this group. Pharmaceutical and device development efforts also prioritize childhood cerebral ALD due to clear endpoints and measurable outcomes. Advocacy and awareness campaigns aimed at pediatric populations enhance diagnostic rates and treatment uptake. Reimbursement policies in major markets often favor aggressive pediatric interventions, reinforcing revenue dominance for this segment. Clinical trial activity focusing on early-onset disease continues to attract investment to childhood cerebral ALD. The overall combination of clinical burden, care intensity, and focused R&D secures this segment’s leading position.
The Adult cerebral ALD segment is anticipated to witness the fastest growth rate of 19.2% from 2025 to 2032, as increasing recognition of late-onset cerebral phenotypes drives diagnosis and treatment uptake. Improved MRI protocols and better clinician awareness are identifying more adult cerebral cases that were previously misdiagnosed as other neurodegenerative disorders. Growing use of VLCFA screening and antibody panels in adult neurology clinics is uncovering a larger patient pool. Advances in disease-modifying therapies and expanded eligibility for transplant or gene therapy in adults are increasing marketable treatment opportunities. Adult patients’ longer disease course also creates demand for chronic care, rehabilitation, and symptomatic management products. The evolution of adult-focused clinical trials and real-world evidence is attracting biotech interest and commercial investment. Health systems adapting to provide lifelong monitoring and treatment for ALD add to service demand. Better survival and improved supportive care expand the addressable market among adults over the forecast period.
- By Symptoms
On the basis of symptoms, the market is segmented into paraparesis, adrenocortical insufficiency, psychiatric disorders, dementia, urinary and genital tract disorders, and others. The Paraparesis segment dominated the largest market revenue share of 34.6% in 2024, reflecting its high prevalence among AMN patients and the chronic care requirements it generates. Paraparesis leads to mobility impairment, long-term physiotherapy, orthotic needs, and frequent outpatient neurology consultations, all of which drive service and product demand. The progressive nature of spastic paraparesis necessitates sustained rehabilitative services and assistive devices, increasing lifetime healthcare expenditures. Specialist clinics and multidisciplinary teams commonly manage paraparesis, concentrating market activity in centers of excellence. The symptom’s measurable impact on function makes it a common endpoint in clinical studies, encouraging therapeutic development targeting mobility. Insurer coverage for durable medical equipment and repeated therapies further supports market revenue from this symptom cluster. Public and private rehabilitation investments in major markets continue to expand capacity for paraparesis care. Epidemiological data and registries that track AMN progression have helped quantify the economic burden tied to paraparesis. The cumulative effect of prevalence, chronicity, and resource intensity cements paraparesis as the dominant symptomatic segment.
The Psychiatric disorders segment is expected to register the fastest CAGR of 18.9% from 2025 to 2032, driven by improved recognition of mood, behavioral and cognitive symptoms in both cerebral and adult phenotypes. Psychiatric manifestations prompt psychiatric referrals, neuropsychological testing, and psycho-pharmacologic treatment, broadening the market for diagnostic and therapeutic services. Growing integration of mental health screening into neurology and oncology clinics is increasing detection rates. The expansion of telepsychiatry and community mental health programs improves access for ALD patients with psychiatric symptoms. Research linking neuroinflammation and psychiatric presentation is stimulating development of targeted neuroprotective and anti-inflammatory treatments. Increased attention to quality-of-life endpoints in trials is also raising clinical focus on psychiatric outcomes. Awareness campaigns and caregiver education reduce stigma and encourage earlier care seeking. These factors collectively accelerate market expansion for psychiatric management in ALD.
- By Treatment
On the basis of treatment, the market is segmented into stem cell transplant, adrenal insufficiency treatment, gene therapy, physical therapy, corticosteroids, Lorenzo’s oil, and others. The Stem cell transplant segment dominated the largest market revenue share of 36.8% in 2024, because hematopoietic stem cell transplantation (HSCT) remains the only established intervention shown to halt cerebral demyelination in early stages. HSCT’s role in altering disease trajectory for appropriately selected childhood cerebral ALD patients makes it a high-value procedure with substantial hospital, transplant unit, and follow-up care revenues. The complexity of transplantation requires specialized multidisciplinary teams, extended inpatient stays, and long-term monitoring, all contributing to significant market spend. Donor sourcing, conditioning regimens, and graft-versus-host disease management add to the service and product mix associated with HSCT. Health systems in developed markets invest in transplant infrastructure and post-transplant rehabilitation, further concentrating market value in this treatment. Clinical guidelines that recommend early transplant for MRI-positive but clinically presymptomatic children underpin steady utilization. Ongoing trial activity exploring optimal timing and conditioning enhances clinician confidence and uptake. The combination of life-altering clinical benefit and high procedural cost secures HSCT’s dominant revenue position.
The Gene therapy segment is anticipated to witness the fastest growth rate of 22.4% from 2025 to 2032, propelled by advances in lentiviral and AAV-based corrective strategies targeting ABCD1 mutations. Promising clinical trial results showing stabilization or improvement in neurological outcomes are attracting major biotech and institutional investment. Regulatory pathways for rare disease gene therapies and potential single-administration treatment models increase the commercial appeal. Gene therapy’s potential to reduce lifelong care needs and transform prognosis is driving payer and investor interest despite high upfront costs. Expanded trial enrollment criteria and compassionate use programs are accelerating real-world evidence generation. Manufacturing scale-up and improved vector delivery technologies are lowering per-patient production hurdles. Strategic partnerships between academic centers and industry are speeding clinical translation. These developments combine to make gene therapy the fastest growing treatment category in the forecast period.
- By Diagnosis
On the basis of diagnosis, the market is segmented into blood testing, MRI, vision screening, skin biopsy and fibroblast cell culture. The MRI segment dominated the largest market revenue share of 45.7% in 2024, because neuroimaging is central to detecting cerebral demyelination, tracking disease progression, and guiding treatment decisions such as HSCT. MRI provides highly sensitive visualization of white-matter changes and contrast enhancement patterns that are diagnostic for cerebral involvement. Routine MRI surveillance in at-risk boys and symptomatic adults creates sustained demand for imaging services and follow-up scans. Advanced protocols, including diffusion and spectroscopy techniques, enhance early detection and prognostication, reinforcing MRI’s clinical importance. The cost and frequency of MRI imaging, especially in tertiary centers, contribute significantly to market revenue. Integration of imaging with multidisciplinary care pathways makes MRI a bottleneck service that drives investment in radiology capacity. Clinical trial endpoints frequently rely on MRI metrics, increasing procedural volumes tied to research activity. The widespread availability of MRI in major markets and its central role in care decisions secure its dominant share.
The Blood Testing segment is expected to witness the fastest growth rate of 20.1% from 2025 to 2032, driven by expansion of newborn and family-screening programs using VLCFA assays and emerging molecular diagnostics. Less invasive, cost-effective blood tests enable broader population screening and earlier diagnosis, increasing the pool of patients entering surveillance pathways. Advances in biochemical and genetic testing, including next-generation sequencing panels for ABCD1, are improving sensitivity and turnaround time. Point-of-care and centralized lab platforms are being adopted in national screening programs, especially where resources permit. Increased awareness and guidelines recommending biochemical screening for at-risk males are fueling demand. The scaling of testing infrastructure and industry efforts to lower per-test costs are accelerating adoption. Blood testing’s scalability and utility across age groups make it the fastest growing diagnostic category.
- By End-Users
On the basis of end-users, the market is segmented into clinics, hospitals, diagnostic centres, and others. The Hospitals segment dominated the largest market revenue share of 49.3% in 2024, reflecting hospitals’ role as primary centers for acute management, HSCT, advanced imaging, and multidisciplinary care for ALD patients. Tertiary hospitals provide integrated services—oncology, neurology, endocrinology, transplant, and rehabilitation—that concentrate high-cost interventions and long-term follow-up within hospital systems. The infrastructure requirements for transplant, complex diagnostics, and inpatient neurologic care channel most revenue through hospital budgets. Hospitals also lead in clinical trial participation and investigator-initiated studies that generate additional service volumes. Reimbursement patterns in major markets favor hospital-based delivery of high-intensity treatments, further cementing dominance. Centres of excellence and referral networks funnel regional cases to specialized hospitals, increasing case volumes and associated revenues. The centralization of expertise and equipment in hospital settings ensures sustained market leadership for this end-user category.
The Diagnostic Centres segment is predicted to grow the fastest at a CAGR of 18.6% from 2025 to 2032, owing to expanded capacity for specialized biochemical assays, genetic testing, and MRI interpretation services. Standalone labs and imaging centers are increasingly partnering with hospitals for outsourced diagnostics, enabling faster access and scalable testing volumes. Investment in high-throughput sequencing, VLCFA platforms, and AI-assisted image reading is expanding diagnostic centre capabilities. Demand for outpatient pre-screening, family testing, and longitudinal monitoring encourages utilization of dedicated diagnostic facilities. Cost efficiencies and faster turnaround times offered by diagnostic centres attract clinicians and patients alike. The growth of tele-diagnostics and remote reporting models further amplifies this segment’s expansion potential.
- By Distribution Channel
On the basis of distribution channel, the market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The Hospital Pharmacy segment dominated the largest market revenue share of 54.1% in 2024, because many ALD therapies and supportive care medications are initiated, administered or monitored within hospital settings. High-value therapies, in-hospital formulations, parenteral supportive care, and transplant-related medicines concentrate procurement within hospital pharmacies. Integrated hospital supply chains, clinician-directed dispensing, and inpatient medication management contribute to the large revenue share. Contracts and bulk purchasing by tertiary centres further reinforce hospital pharmacy prominence. Specialist pharmacy services such as therapeutic drug monitoring and compounding for transplant regimens are typically hospital-based, adding to segment value. The critical nature of many ALD treatments and their initiation in acute settings maintain hospital pharmacy dominance.
The Online Pharmacy segment is expected to register the fastest growth rate of 23.0% from 2025 to 2032, driven by expanding e-commerce penetration, improved cold-chain logistics for specialty products, and patient preference for home delivery of chronic supportive medicines. Online platforms increase geographic access for rare disease patients in underserved regions and provide competitive pricing and subscription refill models. Integration with telehealth and e-prescription systems streamlines repeat dispensing and clinical follow-up. Manufacturer partnerships with e-pharmacies and specialty distributors enhance product availability online. Regulatory acceptance of e-pharmacy models in multiple jurisdictions and improved reimbursement for home delivery are accelerating adoption. These factors combine to make online pharmacy the fastest growing distribution channel through 2032.
X-linked Adrenoleukodystrophy Market Regional Analysis
- North America dominated the X-linked adrenoleukodystrophy market with the largest revenue share of 41.6% in 2024
- Owing to strong healthcare infrastructure, favorable regulatory support for orphan drugs, and the presence of key players such as Bluebird Bio and Minoryx Therapeutics
- The market leads the regional growth, driven by early adoption of gene therapy, rising clinical trial activities, and improved reimbursement policies for rare disease treatments
U.S. X-linked Adrenoleukodystrophy Market Insight
The U.S. X-linked adrenoleukodystrophy market captured the largest revenue share in 2024 within North America, primarily fueled by advanced healthcare infrastructure, widespread availability of specialized treatment centers, and the ongoing development of innovative therapies targeting cerebral and adrenomyeloneuropathy forms of X-ALD. Early adoption of gene therapy, rising clinical trial activities, and improved reimbursement policies for rare disease treatments are further supporting market growth. Increasing patient awareness, newborn screening programs, and proactive diagnostic initiatives are key factors driving the expansion of the U.S. market.
Europe X-linked Adrenoleukodystrophy Market Insight
The Europe X-linked adrenoleukodystrophy market is projected to expand at a substantial CAGR throughout the forecast period, driven by robust healthcare systems, strong emphasis on rare disease research, and increasing availability of advanced therapies. Growing adoption of newborn screening programs and expanding patient registries are further propelling the market.
U.K. X-linked Adrenoleukodystrophy Market Insight
The U.K. X-linked adrenoleukodystrophy market is anticipated to grow at a noteworthy CAGR during the forecast period, owing to the government’s support for rare disease initiatives, increasing clinical trial activity, and rising awareness among healthcare providers about early diagnosis and intervention strategies.
Germany X-linked Adrenoleukodystrophy Market Insight
The Germany X-linked Adrenoleukodystrophy market is expected to expand at a considerable CAGR during the forecast period, driven by strong healthcare infrastructure, advanced diagnostic facilities, and government-backed rare disease programs. Growing adoption of gene therapy trials and research initiatives focused on X-ALD further support the market.
Asia-Pacific X-linked Adrenoleukodystrophy Market Insight
The Asia-Pacific X-linked adrenoleukodystrophy market is projected to be the fastest-growing region during the forecast period (2025–2032), exhibiting a CAGR of 8.9%. Market growth is driven by increasing healthcare expenditure, expanding access to genetic diagnostics, government support for rare disease programs, and rising patient awareness in countries such as China, Japan, and India.
Japan X-linked Adrenoleukodystrophy Market Insight
The Japan X-linked adrenoleukodystrophy market is gaining momentum due to the country’s advanced healthcare ecosystem, strong emphasis on genetic screening, and increasing clinical research activities for rare neurological disorders. Rising public awareness and early intervention programs are key growth drivers.
China X-linked Adrenoleukodystrophy Market Insight
The China X-linked Adrenoleukodystrophy market accounted for the largest revenue share within Asia-Pacific in 2024, driven by government-backed rare disease initiatives, rapid expansion of tertiary hospitals, and technological upgrades in diagnostic and laboratory facilities. Growing patient awareness and increasing access to genetic testing further support market growth.
X-linked Adrenoleukodystrophy Market Share
The X-linked Adrenoleukodystrophy industry is primarily led by well-established companies, including:
- bluebird bio, Inc. (U.S.)
- Minoryx Therapeutics S.L. (Spain)
- Poxel SA (France)
- Viking Therapeutics, Inc. (U.S.)
- Sperogenix Therapeutics (U.S.)
- Autobahn Therapeutics (U.S.)
- NeuroVia Biotech (U.S.)
- MedDay Pharmaceuticals (France)
- Stop ALD Foundation (U.S.)
- Ultragenyx Pharmaceutical Inc. (U.S.)
- Taysha Gene Therapies (U.S.)
- Neurogene Inc. (U.S.)
Latest Developments in Global X-linked Adrenoleukodystrophy Market
- In September 2022, the U.S. Food & Drug Administration (FDA) approved elivaldogene autotemcel (SKYSONA) gene therapy for boys aged 4 to 17 with early, active cerebral ALD (CALD) in cases where a matched stem cell donor is unavailable. This approval represented a major breakthrough, offering the first gene therapy option capable of halting the progression of cerebral demyelination in pediatric patients
- In April 2024, long-term follow-up data were published showing that over 90 percent of patients treated with elivaldogene autotemcel remained free of major functional disabilities six years post-treatment. The findings highlighted the durability of therapeutic effect while noting a small but measurable risk of hematologic malignancies in certain cases, emphasizing the need for ongoing monitoring
- In June 2024, Stanford Medicine became one of the first national sites in the United States to offer SKYSONA infusions, expanding the number of centers capable of delivering this complex therapy and improving patient access across the country
- In February 2025, a study conducted in China reported that optical coherence tomography angiography (OCTA) could detect early retinal microvascular changes in X‑ALD patients. This research suggested potential non-neural biomarkers for early disease progression, providing new avenues for monitoring and diagnosis beyond traditional imaging and biochemical tests
- In May 2025, a Phase 1/2 trial for the gene therapy candidate SBT101, an AAV-based therapy for adult males with the adrenomyeloneuropathy (AMN) phenotype of X‑ALD, reported initial safety and tolerability data. This trial represented a significant step in expanding gene therapy research beyond pediatric cerebral ALD to adult forms of the disease, opening the door for future therapeutic options for a broader patient population
- In August 2025, the FDA approved updates to the labeling of SKYSONA to reflect emerging evidence regarding the small increased risk of hematologic malignancies following treatment. These labeling changes reinforce the importance of long-term patient monitoring and risk management in clinical practice
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.