Middle East And Africa Antibody Drug Conjugates Market
Market Size in USD Million
CAGR :
% 
 USD
413.77 Million
USD
1,180.32 Million
2024
2032
USD
413.77 Million
USD
1,180.32 Million
2024
2032
| 2025 –2032 | |
| USD 413.77 Million | |
| USD 1,180.32 Million | |
|
|
|
|
Middle East and Africa Antibody Drug Conjugates (ADC) Market Size
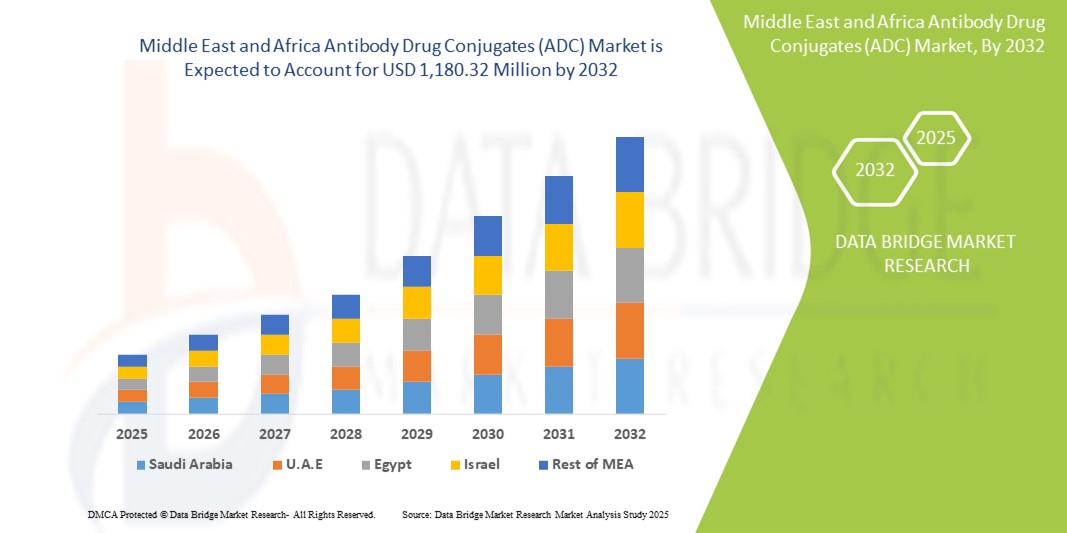
- The Middle East and Africa antibody drug conjugates (ADC) market size was valued at USD 413.77 million in 2024 and is expected to reach USD 1,180.32 million by 2032, at a CAGR of 14.00% during the forecast period
- The market growth is primarily driven by the increasing burden of cancer and the expanding adoption of targeted therapies across healthcare systems in the region
- In addition, rising investments in precision medicine, improving healthcare infrastructure, and growing awareness of advanced biologics are establishing ADCs as a critical component of oncology treatment. These converging factors are accelerating the adoption of ADC therapies, thereby significantly boosting the region’s market expansion
Middle East and Africa Antibody Drug Conjugates (ADC) Market Analysis
- Middle Antibody drug conjugates (ADCs), which combine the targeting ability of monoclonal antibodies with the cytotoxic potency of chemotherapy agents, are gaining traction as a transformative cancer treatment across the Middle East and Africa due to their precision, reduced side effects, and improved clinical outcomes in oncology care
- The growing demand for ADCs is primarily fueled by rising cancer prevalence, increasing focus on personalized medicine, and expanding access to advanced therapeutic options through government and private sector investments
- South Africa dominated the Middle East and Africa antibody drug conjugates (ADC) market in with the largest revenue share of 28.9% in 2024, supported by its advanced healthcare infrastructure, strong oncology research base, and early adoption of biologic therapies through clinical collaborations and public-private partnerships
- Saudi Arabia is expected to be the fastest-growing country in the Middle East and Africa antibody drug conjugates (ADC) market during the forecast period due to accelerated healthcare modernization, rising oncology diagnosis rates, and initiatives to localize pharmaceutical manufacturing and strengthen regulatory approvals for biologics
- The breast cancer segment dominated the Middle East and Africa antibody drug conjugates (ADC) market in the Middle East and Africa with a market share of 40.2% in 2024, driven by its high incidence and increasing deployment of targeted ADC therapies for HER2-positive and triple-negative breast cancer cases across both public and private oncology centers
Report Scope and Middle East and Africa Antibody Drug Conjugates (ADC) Market Segmentation
|
Attributes |
Middle East and Africa Antibody Drug Conjugates (ADC) Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
Middle East and Africa
|
|
Key Market Players |
|
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include in-depth expert analysis, pricing analysis, brand share analysis, consumer survey, demography analysis, supply chain analysis, value chain analysis, raw material/consumables overview, vendor selection criteria, PESTLE Analysis, Porter Analysis, and regulatory framework. |
Middle East and Africa Antibody Drug Conjugates (ADC) Market Trends
“Targeted Therapies Advancing Through Innovation and Localization”
- A significant and accelerating trend in the Middle East and Africa ADC market is the advancement of targeted cancer therapies through innovative ADC research and an increasing push toward local pharmaceutical manufacturing. This shift is enabling greater access to advanced oncology treatments within the region
- For instance, South African biotech firms have begun collaborating with global pharmaceutical companies to establish local clinical trials and technology transfer agreements for next-generation ADCs. Similarly, Saudi Arabia’s Vision 2030 healthcare initiatives aim to bolster domestic pharmaceutical capabilities, including biologics and targeted cancer drugs
- Emerging ADC platforms in the region are leveraging innovations in linker chemistry and site-specific conjugation techniques, improving the therapeutic index and reducing systemic toxicity of treatments. These developments allow healthcare providers to offer safer, more effective cancer therapy options tailored to regional patient needs
- Increasing public-private partnerships are also fueling educational outreach and clinical awareness about ADCs, particularly among oncologists and healthcare administrators, encouraging broader usage and inclusion in national cancer treatment protocols
- This trend of advancing ADC innovation through local capacity-building and international collaboration is fundamentally transforming the oncology landscape in the region. Consequently, companies such as BioCentrix and other regional biotech startups are investing in ADC development pipelines aimed at addressing prevalent cancers such as breast, lung, and hematologic malignancies
- The demand for safer, more targeted cancer therapies such as ADCs is growing rapidly across the Middle East and Africa, driven by an increasing cancer burden and a regional healthcare shift toward biologics and personalized medicine
Middle East and Africa Antibody Drug Conjugates (ADC) Market Dynamics
Driver
“Rising Cancer Incidence and Expansion of Precision Oncology Infrastructure”
- The increasing cancer burden across the Middle East and Africa, coupled with growing investments in oncology-focused precision medicine, is a key driver propelling the regional ADC market
- For instance, in May 2024, the Saudi National Biotechnology Strategy announced support for biologics and ADC development as part of its national cancer treatment initiative. Such programs aim to accelerate access to targeted therapies and improve survival outcomes for patients
- As more healthcare institutions integrate precision oncology tools such as molecular diagnostics and biomarker testing, ADCs are emerging as an effective and preferred treatment modality due to their targeted mechanism and reduced toxicity profiles
- Furthermore, the rising adoption of advanced biologics in countries such as the UAE and South Africa, supported by improved reimbursement frameworks and national cancer registries, is increasing the uptake of ADCs in both public and private healthcare systems
- Government initiatives to expand cancer care access and partnerships with global pharmaceutical leaders are helping to streamline ADC availability in tertiary hospitals, thereby boosting overall market growth in the region
Restraint/Challenge
“Limited Diagnostic Infrastructure and High Therapy Costs”
- Limited access to advanced diagnostic tools and the high cost of ADC therapies pose major challenges to wider market penetration in many parts of the Middle East and Africa
- For instance, in lower-income countries such as Nigeria or Kenya, the absence of widespread HER2 and biomarker testing infrastructure restricts oncologists from prescribing ADCs effectively, limiting patient access to targeted treatment pathways
- Moreover, the high manufacturing cost of ADCs—stemming from complex conjugation processes and stringent regulatory controls—translates to elevated prices for healthcare providers and patients, making reimbursement and affordability a key barrier
- While local governments are introducing oncology access programs, including subsidized care and generic drug development, ADCs remain costly and are often limited to urban centers or private healthcare systems
- Addressing these challenges through regional investment in molecular diagnostics, technology transfer initiatives, local production partnerships, and tiered pricing models will be essential to unlocking the full potential of ADC therapies in the Middle East and Africa
Middle East and Africa Antibody Drug Conjugates (ADC) Market Scope
The market is segmented on the basis of product, antigen component, antibody component, linker component, cytotoxic payloads, linker technology, conjugation technology, indication, end user, and distribution channel.
- By Product
On the basis of product, the Middle East and Africa antibody drug conjugates (ADC) market is segmented into Enhertu, Kadcyla, Trodelvy, Polivy, Adcetris, Padcev, Besponsa, Elahere, Zylonta, Mylotarg, Tivdak, and Others. The Enhertu segment dominated the market with the largest market revenue share in 2024, driven by its strong clinical performance in HER2-positive breast cancer and growing use in gastric and lung cancers. Its success is reinforced by increasing availability in advanced oncology centers and supportive national cancer programs.
The Trodelvy segment is anticipated to witness the fastest growth rate from 2025 to 2032, fueled by rising adoption in treating triple-negative breast cancer and urothelial cancers. Regional trials and strategic access initiatives are expanding its clinical footprint across oncology networks.
- By Antigen Component
On the basis of antigen component, the Middle East and Africa antibody drug conjugates (ADC) market is segmented into HER2 Receptor, Trop-2, CD79B, CD30, Nectin 4, CD22, CD19, CD33, Tissue Factors, and Others. The HER2 receptor segment held the largest market revenue share in 2024, driven by a high prevalence of HER2-positive breast cancer and established use of HER2-targeted ADCs such as Kadcyla and Enhertu. Diagnostic capacity improvements are further supporting segment growth.
The Trop-2 segment is expected to witness the fastest CAGR from 2025 to 2032, driven by its role in aggressive cancers and increased regional uptake of Trodelvy. Advancements in biomarker testing are also aiding clinical identification and treatment matching.
- By Antibody Component
On the basis of antibody component, the Middle East and Africa antibody drug conjugates (ADC) market is segmented into Third Generation ADCs, Second Generation ADCs, Fourth Generation ADCs, and First Generation ADCs. The second generation ADCs segment held the largest market revenue share in 2024, due to their presence in approved products and their balance of efficacy and safety. Drugs such as Kadcyla and Adcetris are key contributors to this segment.
The third generation ADCs segment is expected to witness the fastest CAGR from 2025 to 2032, supported by advances in linker-payload technology and improved targeting precision, enabling stronger therapeutic outcomes and broader clinical applications.
- By Linkers Component
On the basis of linker component, the Middle East and Africa antibody drug conjugates (ADC) market is segmented into cleavable linkers and non cleavable linkers. The cleavable linkers segment dominated the market with the largest revenue share in 2024, as most approved ADCs use this technology to ensure efficient payload release in tumor environments. Their compatibility with acidic and enzymatic conditions strengthens their utility.
The non cleavable linkers segment is projected to grow at the fastest rate during forecast period, due to their higher plasma stability and use in next-generation ADCs, helping improve control over payload delivery and reduce off-target toxicity.
- By Cytotoxic Payloads or Warheads Component
On the basis of cytotoxic payloads, the Middle East and Africa antibody drug conjugates (ADC) market is segmented into DNA damaging agents and microtubule disrupting agents. The microtubule disrupting agents segment held the largest market revenue share in 2024, due to their established use in products such as Kadcyla and Adcetris, which are widely administered across oncology centers.
The DNA damaging agents segment is anticipated to witness the fastest CAGR from 2025 to 2032, supported by their presence in innovative ADCs such as Enhertu and their growing application across a broader range of solid tumors.
- By Linker Technology
On the basis of linker technology, the Middle East and Africa antibody drug conjugates (ADC) market is segmented into peptide linkers, thioether linkers, hydrazone linkers, and disulfide linkers. The Peptide Linkers segment dominated the market with the largest revenue share in 2024, given their widespread adoption in cleavable ADCs and their responsiveness to tumor-specific enzymes.
The thioether linkers segment is projected to witness the fastest growth during forecast period, driven by their chemical stability and growing preference in non-cleavable linker-based ADC platforms to optimize therapeutic index.
- By Conjugation Technology
On the basis of conjugation technology, the Middle East and Africa antibody drug conjugates (ADC) market is segmented into site-specific conjugation and chemical conjugation. The Chemical Conjugation segment held the largest market revenue share in 2024, due to its use in earlier ADC generations and commercial-scale manufacturing methods.
The site-specific conjugation segment is expected to register the fastest growth from 2025 to 2032, driven by the ability to produce more uniform ADC products with precise drug-to-antibody ratios, improving efficacy and safety profiles.
- By Indication
On the basis of indication, the Middle East and Africa antibody drug conjugates (ADC) market is segmented into breast cancer, blood cancer (leukemia, lymphoma), lung cancer, gynecological cancer, gastrointestinal cancer, genitourinary cancer, and others. The breast cancer segment dominated the market with a market share of 40.2% in 2024, owing to its high regional prevalence and widespread adoption of HER2- and Trop-2-targeted ADCs such as Enhertu and Trodelvy.
The Lung Cancer segment is expected to witness the fastest growth rate from 2025 to 2032, fueled by rising diagnosis rates and emerging ADC therapies targeting HER2 and other biomarkers.
- By End User
On the basis of end user, the Middle East and Africa antibody drug conjugates (ADC) market is segmented into hospitals, specialty center, clinics, ambulatory centers, home healthcare, and others. The Hospitals segment held the largest market revenue share in 2024, supported by advanced oncology infrastructure, centralized treatment systems, and access to high-cost ADC therapies in both public and private hospital settings.
The Specialty Center segment is expected to witness the fastest CAGR from 2025 to 2032, as cancer-focused institutions expand across the region and offer targeted biologics with expert care and diagnostic integration.
- By Distribution Channel
On the basis of distribution channel, the Middle East and Africa antibody drug conjugates (ADC) market is segmented into direct tenders, retail sales, and others. The direct tenders segment dominated the market with the largest revenue share in 2024, driven by government procurement of high-cost oncology drugs through centralized hospital supply chains.
The retail sales segment is projected to grow at the fastest rate during the forecast period due to the increasing distribution of ADCs through specialty and hospital-linked pharmacies, particularly in the private healthcare sector
Middle East and Africa Antibody Drug Conjugates (ADC) Market Regional Analysis
- South Africa dominated the antibody drug conjugates (ADC) market in the Middle East and Africa with the largest revenue share of 28.9% in 2024, supported by its advanced healthcare infrastructure, strong oncology research base, and early adoption of biologic therapies through clinical collaborations and public-private partnerships
- Patients and healthcare providers in the country increasingly prefer ADCs for their precision, reduced toxicity, and clinical effectiveness in treating aggressive cancers such as breast and lymphoma, especially in tertiary care settings
- This rising adoption is further supported by active participation in global clinical trials, regional distribution agreements, and government-led initiatives aimed at expanding oncology care, positioning ADCs as a critical component of advanced cancer treatment strategies in South Africa
The South Africa Antibody Drug Conjugates (ADC) Market Insight
The South Africa antibody drug conjugates (ADC) market captured the largest revenue share in the Middle East and Africa region in 2024, driven by the country’s relatively advanced oncology infrastructure and growing investment in targeted cancer therapies. The presence of specialized cancer centers, coupled with improved access to biologics, supports the widespread use of ADCs in breast and blood cancers. In addition, collaborative clinical trials with international biopharma companies and strong government efforts to expand oncology services are significantly contributing to market expansion.
Saudi Arabia Antibody Drug Conjugates (ADC) Market Insight
The Saudi Arabia antibody drug conjugates (ADC) market is projected to grow at a substantial CAGR during the forecast period, supported by national healthcare transformation efforts under Vision 2030 and increasing focus on precision medicine. With a rising cancer burden and high investment in biotechnology and pharmaceutical innovation, ADCs are gaining traction across major hospitals and research institutions. Government initiatives to localize drug manufacturing and strengthen regulatory approvals for advanced biologics are further accelerating market growth.
United Arab Emirates (UAE) Antibody Drug Conjugates (ADC) Market Insight
The UAE antibody drug conjugates (ADC) market is anticipated to grow at a noteworthy CAGR, fueled by the country’s strong emphasis on healthcare digitization and innovation in cancer treatment. The rapid expansion of oncology centers, medical tourism, and partnerships with international pharmaceutical firms are facilitating ADC adoption. With a tech-forward healthcare system and growing awareness of targeted therapies among clinicians, ADCs are increasingly integrated into clinical protocols for hard-to-treat cancers.
Middle East and Africa Antibody Drug Conjugates (ADC) Market Share
The Middle East and Africa antibody drug conjugates (ADC) industry is primarily led by well-established companies, including:
- F. Hoffmann-La Roche Ltd (Switzerland)
- AstraZeneca (U.K.)
- Pfizer Inc. (U.S.)
- Daiichi Sankyo Co., Ltd. (Japan)
- Gilead Sciences, Inc. (U.S.)
- Takeda Pharmaceutical Company Limited (Japan)
- AbbVie Inc. (U.S.)
- GSK plc (U.K.)
- ImmunoGen, Inc. (U.S.)
- ADC Therapeutics SA (Switzerland)
- Synaffix B.V. (Netherlands)
- Lonza Group AG (Switzerland)
- Samsung Biologics Co., Ltd. (South Korea)
- Catalent, Inc. (U.S.)
- WuXi Biologics (China)
- Mablink Bioscience (France)
- Mersana Therapeutics, Inc. (U.S.)
- Sutro Biopharma, Inc. (U.S.)
- Julphar (Gulf Pharmaceutical Industries) (U.A.E)
What are the Recent Developments in Global Middle East and Africa Antibody Drug Conjugates (ADC) Market?
- In April 2024, Riyadh Pharma, a leading Saudi-based pharmaceutical manufacturer, signed a strategic collaboration with a global biotech firm to establish local production capabilities for antibody drug conjugates. This initiative marks a significant step in enhancing regional biopharmaceutical manufacturing and improving access to targeted cancer therapies across the Middle East. The move supports Saudi Arabia’s Vision 2030 goals to localize advanced drug technologies and strengthen oncology care infrastructure
- In March 2024, The South African Medical Research Council (SAMRC) announced the launch of a national oncology research program focused on advanced biologics, including ADCs. This initiative aims to support clinical trials, build diagnostic capacity for precision medicine, and expand access to innovative cancer treatments. The program underscores the government's commitment to advancing cancer care and fostering public-private research partnerships
- In February 2024, BioCentrix Africa, a Johannesburg-based biotechnology firm, secured funding for a multi-phase clinical trial of its next-generation HER2-targeting ADC candidate. The trial, conducted in collaboration with academic medical centers, aims to evaluate the safety and efficacy of this therapy in treating metastatic breast cancer. This development demonstrates the growing R&D landscape for ADCs within the African continent and the region's shift toward homegrown biotech innovation
- In January 2024, Egypt’s Ministry of Health partnered with a European oncology consortium to introduce ADCs into select public cancer hospitals under a pilot access program. The partnership includes the establishment of training programs for oncologists and pathologists, as well as capacity-building for HER2 and Trop-2 diagnostic testing. The initiative is expected to expand precision treatment options for underserved populations in Egypt
- In December 2023, Gulf Pharmaceutical Industries (Julphar) announced the formation of a strategic alliance with a leading Asian biosimilar developer to explore the co-development of biosimilar ADCs for hematologic cancers. This collaboration is focused on technology transfer, regulatory alignment, and affordability, aiming to make high-cost cancer therapies more accessible across the Gulf Cooperation Council (GCC) region
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET APPLICATION COVERAGE GRID
2.8 PRODUCT LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTAL ANALYSIS
4.2 PORTER’S FIVE FORCES
4.3 PESTEL ANALYSIS
5 COST STRUCTURE ANALYSIS OF ANTIBODY-DRUG CONJUGATE (ADC) MANUFACTURING
5.1 ANTIBODIES
5.1.1 OVERVIEW OF ANTIBODY PRODUCTION
5.1.1.1 In-house vs. Outsourced:
5.1.2 ANTIBODY PRICING FACTORS
5.2 LINKERS
5.2.1 ROLE AND TYPES OF LINKERS
5.2.1.1 Cost Impact by Linker Type:
5.3 CYTOTOXIC AGENTS
5.3.1 COST CONSIDERATIONS:
5.3.2 BUFFERS AND SOLVENTS
5.4 COST BREAKDOWN BY MANUFACTURING STAGE
5.4.1 PRE-PRODUCTION COSTS
5.4.2 CONJUGATION PROCESS
5.4.3 PURIFICATION AND FILTRATION
5.4.4 QUALITY CONTROL
5.5 COST PROJECTIONS AND PRICING TRENDS (2024–2030)
5.5.1 PROJECTED COST FLUCTUATIONS
5.5.2 COST IMPACT OF SCALABILITY
5.6 SUPPLIER AND GEOGRAPHIC PRICING TRENDS
5.6.1 GEOGRAPHIC COST VARIATIONS
5.6.2 SUPPLIER ANALYSIS
5.6.3 CONCLUSION
6 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: REGULATIONS
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 INCREASING PREVALENCE OF CANCER
7.1.2 ADVANCES IN ANTIBODY-DRUG CONJUGATE (ADC) TECHNOLOGY
7.1.3 INCREASING DEMAND FOR TARGETED THERAPIES
7.1.4 ADVANCEMENTS IN PROTEOMICS AND GENOMICS RESEARCH
7.2 RESTRAINTS
7.2.1 HIGH DEVELOPMENT COST & MANUFACTURING COMPLEXITIES
7.2.2 SAFETY AND TOXICITY ISSUES OF ANTIBODY DRUG CONJUGATES
7.3 OPPORTUNITIES
7.3.1 GROWING ONCOLOGY PIPELINE FOR ANTIBODY DRUG CONJUGATES (ADCS)
7.3.2 INCREASING INVESTMENT IN CANCER RESEARCH
7.3.3 INCREASING COLLABORATION WITH RESEARCH INSTITUTIONS FOR ANTIBODY DRUG CONJUGATES
7.4 CHALLENGES
7.4.1 CLINICAL TRIAL FAILURES FOR ANTIBODY DRUG CONJUGATES DEVELOPMENT
7.4.2 LENGTHY CLINICAL TRIALS AND DEVELOPMENT PHASES
8 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT
8.1 OVERVIEW
8.2 ENHERTU
8.3 KADCYLA
8.4 TRODELVY
8.5 POLIVY
8.6 ADCETRIS
8.7 PADCEV
8.8 BESPONSA
8.9 ELAHERE
8.1 ZYLONTA
8.11 MYLOTARG
8.12 TIVDAK
8.13 OTHERS
9 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY ANTIGEN COMPONENT
9.1 OVERVIEW
9.2 HER2 RECEPTOR
9.3 TROP-2
9.4 CD79B
9.5 CD30
9.6 NECTIN 4
9.7 CD22
9.8 CD19
9.9 CD33
9.1 TISSUE FACTORS
9.11 OTHERS
10 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY ANTIBODY COMPONENT
10.1 OVERVIEW
10.2 THIRD GENERATION ADCS
10.3 SECOND GENERATION ADCS
10.4 FOURTH GENERATION ADCS
10.5 FIRST GENERATION ADCS
11 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY LINKERS COMPONENT
11.1 OVERVIEW
11.2 CLEAVABLE LINKERS
11.2.1 PEPTIDE BASED
11.2.2 ACID SENSITIVE OR ACID LABILE
11.2.3 GLUTATHIONE SENSITIVE DISULFIDE
11.3 NON CLEAVABLE LINKERS
12 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY CYTOTOXIC PAYLOADS OR WARHEADS COMPONENT
12.1 OVERVIEW
12.2 DNA DAMAGING AGENTS
12.2.1 CAMPTOTHECIN
12.2.2 CALICHEAMICIN
12.2.3 PYRROLOBENZODIAZEPINES
12.3 MICROTUBULE DISRUPTING AGENTS
12.3.1 AURISTATIN
12.3.2 MAYTANSINOIDS
13 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY LINKER TECHNOLOGY
13.1 OVERVIEW
13.2 PEPTIDE LINKERS
13.3 THIOETHER LINKERS
13.4 HYDRAZONE LINKERS
13.5 DISULFIDE LINKERS
14 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY CONJUGATION TECHNOLOGY
14.1 OVERVIEW
14.2 SITE-SPECIFIC CONJUGATION
14.3 CHEMICAL CONJUGATION
15 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY INDICATION
15.1 OVERVIEW
15.2 BREAST CANCER
15.3 BLOOD CANCER (LEUKEMIA, LYMPHOMA)
15.4 LUNG CANCER
15.5 GYNECOLOGICAL CANCER
15.6 GASTROINTESTINAL CANCER
15.7 GENITOURINARY CANCER
15.8 OTHERS
16 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY END USER
16.1 OVERVIEW
16.2 HOSPITALS
16.3 SPECIALTY CENTER
16.4 CLINICS
16.5 AMBULATORY CENTERS
16.6 HOME HEALTHCARE
16.7 OTHERS
17 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY DISTRIBUTION CHANNEL
17.1 OVERVIEW
17.2 DIRECT TENDERS
17.3 RETAIL SALES
17.3.1 HOSPITAL PHARMACY
17.3.2 RETAIL PHARMACY
17.3.3 ONLINE PHARMACY
17.4 OTHERS
18 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION
18.1 MIDDLE EAST AND AFRICA
18.1.1 SAUDI ARABIA
18.1.2 U.A.E
18.1.3 ISRAEL
18.1.4 SOUTH AFRICA
18.1.5 REST OF MIDDLE EAST AND AFRICA
19 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC): COMPANY LANDSCAPE
19.1 COMPANY SHARE ANALYSIS: MIDDLE EAST AND AFRICA
20 SWOT ANALYSIS
21 COMPANY PROFILES
21.1 DAIICHI SANKYO, INC.
21.1.1 COMPANY SNAPSHOT
21.1.2 REVENUE ANALYSIS
21.1.3 PRODUCT PORTFOLIO
21.1.4 RECENT DEVELOPMENT
21.2 F. HOFFMANN-LA ROCHE LTD
21.2.1 COMPANY SNAPSHOT
21.2.2 REVENUE ANALYSIS
21.2.3 PRODUCT PORTFOLIO
21.2.4 RECENT DEVELOPMENT
21.3 GILEAD SCIENCES, INC.
21.3.1 COMPANY SNAPSHOT
21.3.2 REVENUE
21.3.3 PRODUCT PORTFOLIO
21.3.4 RECENT DEVELOPMENT
21.4 ASTELLAS PHARMA INC.
21.4.1 COMPANY SNAPSHOT
21.4.2 REVENUE ANALYSIS
21.4.3 PRODUCT PORTFOLIO
21.4.4 RECENT DEVELOPMENT
21.5 TAKEDA PHARMACEUTICAL COMPANY LIMITED
21.5.1 COMPANY SNAPSHOT
21.5.2 REVENUE ANALYSIS
21.5.3 PRODUCT PORTFOLIO
21.5.4 RECENT DEVELOPMENT
21.6 ABBVIE INC.
21.6.1 COMPANY SNAPSHOT
21.6.2 REVENUE
21.6.3 PRODUCT PORTFOLIO
21.6.4 RECENT DEVELOPMENT
21.7 ADC THERAPEUTICS SA
21.7.1 6.1 COMPANY SNAPSHOT
21.7.2 REVENUE ANALYSIS
21.7.3 PRODUCT PORTFOLIO
21.7.4 RECENT DEVELOPMENT
21.8 AMGEN, INC.
21.8.1 COMPANY SNAPSHOT
21.8.2 REVENUE ANALYSIS
21.8.3 PRODUCT PORTFOLIO
21.8.4 RECENT DEVELOPMENT
21.9 ASTRAZENECA
21.9.1 COMPANY SNAPSHOT
21.9.2 REVENUE ANALYSIS
21.9.3 PRODUCT PORTFOLIO
21.9.4 RECENT DEVELOPMENT
21.1 BAYER
21.10.1 COMPANY SNAPSHOT
21.10.2 REVENUE ANALYSIS
21.10.3 PRODUCT PORTFOLIO
21.10.4 RECENT DEVELOPMENT
21.11 BYONDIS
21.11.1 COMPANY SNAPSHOT
21.11.2 PRODUCT PORTFOLIO
21.11.3 RECENT DEVELOPMENT
21.12 EISAI INC
21.12.1 COMPANY SNAPSHOT
21.12.2 REVENUE ANALYSIS
21.12.3 PRODUCT PORTFOLIO
21.12.4 RECENT DEVELOPMENT
21.13 GSK PLC
21.13.1 COMPANY SNAPSHOT
21.13.2 REVENUE ANALYSIS
21.13.3 PRODUCT PORTFOLIO
21.13.4 RECENT DEVELOPMENT
21.14 JOHNSON & JOHNSON SERVICES, INC.
21.14.1 COMPANY SNAPSHOT
21.14.2 REVENUE ANALYSIS
21.14.3 PRODUCT PORTFOLIO
21.14.4 RECENT DEVELOPMENT
21.15 OXFORD BIOTHERAPEUTICS
21.15.1 COMPANY SNAPSHOT
21.15.2 PRODUCT PORTFOLIO
21.15.3 RECENT DEVELOPMENT
21.16 PFIZER INC.
21.16.1 COMPANY SNAPSHOT
21.16.2 REVENUE ANALYSIS
21.16.3 PRODUCT PORTFOLIO
21.16.4 RECENT UPDATES
21.17 REMEGEN
21.17.1 COMPANY SNAPSHOT
21.17.2 PRODUCT PORTFOLIO
21.17.3 RECENT DEVELOPMENTS
21.18 SANOFI
21.18.1 COMPANY SNAPSHOT
21.18.2 REVENUE ANALYSIS
21.18.3 PRODUCT PORTFOLIO
21.18.4 RECENT DEVELOPMENT
21.19 SUTRO BIOPHARMA, INC.
21.19.1 COMPANY SNAPSHOT
21.19.2 REVENUE ANALYSIS
21.19.3 PRODUCT PORTFOLIO
21.19.4 RECENT UPDATES
22 QUESTIONNAIRE
23 RELATED REPORTS
List of Table
TABLE 1 PROJECTED PRICE CHANGE (2024–2030)
TABLE 2 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 3 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT, 2022-2031 (VOLUME IN UNITS)
TABLE 4 MIDDLE EAST AND AFRICA ENHERTU IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 5 MIDDLE EAST AND AFRICA KADCYLA IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 6 MIDDLE EAST AND AFRICA TRODELVY IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 7 MIDDLE EAST AND AFRICA POLIVY IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 8 MIDDLE EAST AND AFRICA ADCETRIS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 9 MIDDLE EAST AND AFRICA PADCEV IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 10 MIDDLE EAST AND AFRICA BESPONSA IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 11 MIDDLE EAST AND AFRICA ELAHERE IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 12 MIDDLE EAST AND AFRICA ZYLONTA IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 13 MIDDLE EAST AND AFRICA MYLOTARG IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 14 MIDDLE EAST AND AFRICA TIVDAK IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 15 MIDDLE EAST AND AFRICA OTHERS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 16 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY ANTIGEN COMPONENT, 2022-2031 (USD MILLION)
TABLE 17 MIDDLE EAST AND AFRICA HER2 RECEPTOR IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 18 MIDDLE EAST AND AFRICA TROP-2 IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 19 MIDDLE EAST AND AFRICA CD79B IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 20 MIDDLE EAST AND AFRICA CD30 IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 21 MIDDLE EAST AND AFRICA NECTIN 4 IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 22 MIDDLE EAST AND AFRICA CD22 IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 23 MIDDLE EAST AND AFRICA CD19 IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 24 MIDDLE EAST AND AFRICA CD33 IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 25 MIDDLE EAST AND AFRICA TISSUE FACTORS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 26 MIDDLE EAST AND AFRICA OTHERS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 27 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY ANTIBODY COMPONENT, 2022-2031 (USD MILLION)
TABLE 28 MIDDLE EAST AND AFRICA THIRD GENERATION ADCS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 29 MIDDLE EAST AND AFRICA SECOND GENERATION ADCS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 30 MIDDLE EAST AND AFRICA FOURTH GENERATION ADCS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 31 MIDDLE EAST AND AFRICA FIRST GENERATION ADCS IN OPHTHALMOLOGY MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 32 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY LINKERS COMPONENT, 2022-2031 (USD MILLION)
TABLE 33 MIDDLE EAST AND AFRICA CLEAVABLE LINKERS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 34 MIDDLE EAST AND AFRICA CLEAVABLE LINKERS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY COMPONENT, 2022-2031 (USD MILLION)
TABLE 35 MIDDLE EAST AND AFRICA NON CLEAVABLE LINKERS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 36 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY CYTOTOXIC PAYLOADS OR WARHEADS COMPONENT, 2022-2031 (USD MILLION)
TABLE 37 MIDDLE EAST AND AFRICA DNA DAMAGING AGENTS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 38 MIDDLE EAST AND AFRICA DNA DAMAGING AGENTS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY COMPONENT, 2022-2031 (USD MILLION)
TABLE 39 MIDDLE EAST AND AFRICA MICROTUBULE DISRUPTING AGENTS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 40 MIDDLE EAST AND AFRICA MICROTUBULE DISRUPTING AGENTS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY COMPONENT, 2022-2031 (USD MILLION)
TABLE 41 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY LINKER TECHNOLOGY, 2022-2031 (USD MILLION)
TABLE 42 MIDDLE EAST AND AFRICA PEPTIDE LINKERS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 43 MIDDLE EAST AND AFRICA THIOETHER LINKERS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 44 MIDDLE EAST AND AFRICA HYDRAZONE LINKERS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 45 MIDDLE EAST AND AFRICA DISULFIDE LINKERS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 46 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY CONJUGATION TECHNOLOGY, 2022-2031 (USD MILLION)
TABLE 47 MIDDLE EAST AND AFRICA SITE-SPECIFIC CONJUGATION IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 48 MIDDLE EAST AND AFRICA CHEMICAL CONJUGATION IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 49 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY INDICATION, 2022-2031 (USD MILLION)
TABLE 50 MIDDLE EAST AND AFRICA BREAST CANCER IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 51 MIDDLE EAST AND AFRICA BLOOD CANCER (LEUKEMIA, LYMPHOMA) IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 52 MIDDLE EAST AND AFRICA LUNG CANCER IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 53 MIDDLE EAST AND AFRICA GYNECOLOGICAL CANCER IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 54 MIDDLE EAST AND AFRICA GASTROINTESTINAL CANCER IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 55 MIDDLE EAST AND AFRICA GENITOURINARY CANCER IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 56 MIDDLE EAST AND AFRICA OTHERS IN OPHTHALMOLOGY MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 57 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 58 MIDDLE EAST AND AFRICA HOSPITALS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 59 MIDDLE EAST AND AFRICA SPECIALTY CENTERS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 60 MIDDLE EAST AND AFRICA CLINICS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 61 MIDDLE EAST AND AFRICA AMBULATORY CENTERS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 62 MIDDLE EAST AND AFRICA HOME HEALTHCARE IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 63 MIDDLE EAST AND AFRICA OTHERS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 64 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 65 MIDDLE EAST AND AFRICA DIRECT TENDERS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 66 MIDDLE EAST AND AFRICA RETAIL SALES IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 67 MIDDLE EAST AND AFRICA RETAIL SALES IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 68 MIDDLE EAST AND AFRICA OTHERS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY REGION, 2022-2031 (USD MILLION)
TABLE 69 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY COUNTRY, 2022-2031 (USD MILLION)
TABLE 70 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 71 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT, 2022-2031 (VOLUME IN UNITS)
TABLE 72 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT, 2022-2031 (ASP)
TABLE 73 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY ANTIGEN COMPONENT, 2022-2031 (USD MILLION)
TABLE 74 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY ANTIBODY COMPONENT, 2022-2031 (USD MILLION)
TABLE 75 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY LINKERS COMPONENT, 2022-2031 (USD MILLION)
TABLE 76 MIDDLE EAST AND AFRICA CLEAVABLE LINKERS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY COMPONENT, 2022-2031 (USD MILLION)
TABLE 77 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY CYTOTOXIC PAYLOADS OR WARHEADS COMPONENT, 2022-2031 (USD MILLION)
TABLE 78 MIDDLE EAST AND AFRICA DNA DAMAGING AGENTS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY COMPONENT, 2022-2031 (USD MILLION)
TABLE 79 MIDDLE EAST AND AFRICA MICROTUBULE DISRUPTING AGENTS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY COMPONENT, 2022-2031 (USD MILLION)
TABLE 80 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY LINKER TECHNOLOGY, 2022-2031 (USD MILLION)
TABLE 81 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY CONJUGATION TECHNOLOGY, 2022-2031 (USD MILLION)
TABLE 82 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY INDICATION, 2022-2031 (USD MILLION)
TABLE 83 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 84 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 85 MIDDLE EAST AND AFRICA RETAIL SALES IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 86 SAUDI ARABIA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 87 SAUDI ARABIA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT, 2022-2031 (VOLUME IN UNITS)
TABLE 88 SAUDI ARABIA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT, 2022-2031 (ASP)
TABLE 89 SAUDI ARABIA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY ANTIGEN COMPONENT, 2022-2031 (USD MILLION)
TABLE 90 SAUDI ARABIA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY ANTIBODY COMPONENT, 2022-2031 (USD MILLION)
TABLE 91 SAUDI ARABIA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY LINKERS COMPONENT, 2022-2031 (USD MILLION)
TABLE 92 SAUDI ARABIA CLEAVABLE LINKERS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY COMPONENT, 2022-2031 (USD MILLION)
TABLE 93 SAUDI ARABIA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY CYTOTOXIC PAYLOADS OR WARHEADS COMPONENT, 2022-2031 (USD MILLION)
TABLE 94 SAUDI ARABIA DNA DAMAGING AGENTS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY COMPONENT, 2022-2031 (USD MILLION)
TABLE 95 SAUDI ARABIA MICROTUBULE DISRUPTING AGENTS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY COMPONENT, 2022-2031 (USD MILLION)
TABLE 96 SAUDI ARABIA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY LINKER TECHNOLOGY, 2022-2031 (USD MILLION)
TABLE 97 SAUDI ARABIA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY CONJUGATION TECHNOLOGY, 2022-2031 (USD MILLION)
TABLE 98 SAUDI ARABIA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY INDICATION, 2022-2031 (USD MILLION)
TABLE 99 SAUDI ARABIA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 100 SAUDI ARABIA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 101 SAUDI ARABIA RETAIL SALES IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 102 U.A.E. ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 103 U.A.E. ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT, 2022-2031 (VOLUME IN UNITS)
TABLE 104 U.A.E. ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT, 2022-2031 (ASP)
TABLE 105 U.A.E. ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY ANTIGEN COMPONENT, 2022-2031 (USD MILLION)
TABLE 106 U.A.E. ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY ANTIBODY COMPONENT, 2022-2031 (USD MILLION)
TABLE 107 U.A.E. ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY LINKERS COMPONENT, 2022-2031 (USD MILLION)
TABLE 108 U.A.E. CLEAVABLE LINKERS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY COMPONENT, 2022-2031 (USD MILLION)
TABLE 109 U.A.E. ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY CYTOTOXIC PAYLOADS OR WARHEADS COMPONENT, 2022-2031 (USD MILLION)
TABLE 110 U.A.E. DNA DAMAGING AGENTS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY COMPONENT, 2022-2031 (USD MILLION)
TABLE 111 U.A.E. MICROTUBULE DISRUPTING AGENTS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY COMPONENT, 2022-2031 (USD MILLION)
TABLE 112 U.A.E. ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY LINKER TECHNOLOGY, 2022-2031 (USD MILLION)
TABLE 113 U.A.E. ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY CONJUGATION TECHNOLOGY, 2022-2031 (USD MILLION)
TABLE 114 U.A.E. ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY INDICATION, 2022-2031 (USD MILLION)
TABLE 115 U.A.E. ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 116 U.A.E. ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 117 U.A.E. RETAIL SALES IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 118 ISRAEL ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 119 ISRAEL ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT, 2022-2031 (VOLUME IN UNITS)
TABLE 120 ISRAEL ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT, 2022-2031 (ASP)
TABLE 121 ISRAEL ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY ANTIGEN COMPONENT, 2022-2031 (USD MILLION)
TABLE 122 ISRAEL ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY ANTIBODY COMPONENT, 2022-2031 (USD MILLION)
TABLE 123 ISRAEL ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY LINKERS COMPONENT, 2022-2031 (USD MILLION)
TABLE 124 ISRAEL CLEAVABLE LINKERS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY COMPONENT, 2022-2031 (USD MILLION)
TABLE 125 ISRAEL ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY CYTOTOXIC PAYLOADS OR WARHEADS COMPONENT, 2022-2031 (USD MILLION)
TABLE 126 ISRAEL MICROTUBULE DISRUPTING AGENTS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY COMPONENT, 2022-2031 (USD MILLION)
TABLE 127 ISRAEL ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY LINKER TECHNOLOGY, 2022-2031 (USD MILLION)
TABLE 128 ISRAEL ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY CONJUGATION TECHNOLOGY, 2022-2031 (USD MILLION)
TABLE 129 ISRAEL ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY INDICATION, 2022-2031 (USD MILLION)
TABLE 130 ISRAEL ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 131 ISRAEL ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 132 ISRAEL RETAIL SALES IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 133 SOUTH AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 134 SOUTH AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT, 2022-2031 (VOLUME IN UNITS)
TABLE 135 SOUTH AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT, 2022-2031 (ASP)
TABLE 136 SOUTH AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY ANTIGEN COMPONENT, 2022-2031 (USD MILLION)
TABLE 137 SOUTH AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY ANTIBODY COMPONENT, 2022-2031 (USD MILLION)
TABLE 138 SOUTH AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY LINKERS COMPONENT, 2022-2031 (USD MILLION)
TABLE 139 SOUTH AFRICA CLEAVABLE LINKERS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY COMPONENT, 2022-2031 (USD MILLION)
TABLE 140 SOUTH AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY CYTOTOXIC PAYLOADS OR WARHEADS COMPONENT, 2022-2031 (USD MILLION)
TABLE 141 SOUTH AFRICA DNA DAMAGING AGENTS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY COMPONENT, 2022-2031 (USD MILLION)
TABLE 142 SOUTH AFRICA MICROTUBULE DISRUPTING AGENTS IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY COMPONENT, 2022-2031 (USD MILLION)
TABLE 143 SOUTH AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY LINKER TECHNOLOGY, 2022-2031 (USD MILLION)
TABLE 144 SOUTH AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY CONJUGATION TECHNOLOGY, 2022-2031 (USD MILLION)
TABLE 145 SOUTH AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY INDICATION, 2022-2031 (USD MILLION)
TABLE 146 SOUTH AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 147 SOUTH AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 148 SOUTH AFRICA RETAIL SALES IN ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 149 REST OF MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 150 REST OF MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT, 2022-2031 (VOLUME IN UNITS)
TABLE 151 REST OF MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET, BY PRODUCT, 2022-2031 (ASP)
List of Figure
FIGURE 1 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: MIDDLE EAST AND AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 8 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: DBMR MARKET POSITION GRID
FIGURE 9 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: SEGMENTATION
FIGURE 11 EXECUTIVE SUMMARY
FIGURE 12 STRATEGIC DECISIONS
FIGURE 13 RISING INCIDENCE OF CANCER IS DRIVING THE GROWTH OF THE MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET FROM 2024 TO 2031
FIGURE 14 THE PRODUCT SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET IN 2024 AND 2031
FIGURE 15 DROC
FIGURE 16 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY PRODUCT, 2023
FIGURE 17 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY PRODUCT, 2024-2031 (USD MILLION)
FIGURE 18 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY PRODUCT, CAGR (2024-2031)
FIGURE 19 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 20 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY ANTIGEN COMPONENT, 2023
FIGURE 21 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY ANTIGEN COMPONENT, 2024-2031 (USD MILLION)
FIGURE 22 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY ANTIGEN COMPONENT, CAGR (2024-2031)
FIGURE 23 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY ANTIGEN COMPONENT, LIFELINE CURVE
FIGURE 24 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY ANTIBODY COMPONENT, 2023
FIGURE 25 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY ANTIBODY COMPONENT, 2024-2031 (USD MILLION)
FIGURE 26 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY ANTIBODY COMPONENT, CAGR (2024-2031)
FIGURE 27 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY ANTIBODY COMPONENT, LIFELINE CURVE
FIGURE 28 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY LINKERS COMPONENT, 2023
FIGURE 29 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY LINKERS COMPONENT, 2024-2031 (USD MILLION)
FIGURE 30 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY LINKERS COMPONENT, CAGR (2024-2031)
FIGURE 31 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY LINKERS COMPONENT, LIFELINE CURVE
FIGURE 32 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY CYTOTOXIC PAYLOADS OR WARHEADS COMPONENT, 2023
FIGURE 33 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY CYTOTOXIC PAYLOADS OR WARHEADS COMPONENT, 2024-2031 (USD MILLION)
FIGURE 34 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY CYTOTOXIC PAYLOADS OR WARHEADS COMPONENT, CAGR (2024-2031)
FIGURE 35 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY CYTOTOXIC PAYLOADS OR WARHEADS COMPONENT, LIFELINE CURVE
FIGURE 36 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY LINKER TECHNOLOGY, 2023
FIGURE 37 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY LINKER TECHNOLOGY, 2024-2031 (USD MILLION)
FIGURE 38 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY LINKER TECHNOLOGY, CAGR (2024-2031)
FIGURE 39 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY LINKER TECHNOLOGY, LIFELINE CURVE
FIGURE 40 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY CONJUGATION TECHNOLOGY, 2023
FIGURE 41 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY CONJUGATION TECHNOLOGY, 2024-2031 (USD MILLION)
FIGURE 42 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY CONJUGATION TECHNOLOGY, CAGR (2024-2031)
FIGURE 43 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY CONJUGATION TECHNOLOGY, LIFELINE CURVE
FIGURE 44 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY INDICATION, 2023
FIGURE 45 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY INDICATION, 2024-2031 (USD MILLION)
FIGURE 46 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY INDICATION, CAGR (2024-2031)
FIGURE 47 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY INDICATION, LIFELINE CURVE
FIGURE 48 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY END USER, 2023
FIGURE 49 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY END USER, 2024-2031 (USD MILLION)
FIGURE 50 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY END USER, CAGR (2024-2031)
FIGURE 51 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY END USER, LIFELINE CURVE
FIGURE 52 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY DISTRIBUTION CHANNEL, 2023
FIGURE 53 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY DISTRIBUTION CHANNEL, 2024-2031 (USD MILLION)
FIGURE 54 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2024-2031)
FIGURE 55 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 56 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC) MARKET: SNAPSHOT (2023)
FIGURE 57 MIDDLE EAST AND AFRICA ANTIBODY DRUG CONJUGATES (ADC): COMPANY SHARE 2023 (%)

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.