Middle East And Africa Capillary Blood Collection And Sampling Devices Market
Market Size in USD Billion
CAGR :
% 
 USD
28.49 Billion
USD
38.99 Billion
2022
2030
USD
28.49 Billion
USD
38.99 Billion
2022
2030
| 2023 –2030 | |
| USD 28.49 Billion | |
| USD 38.99 Billion | |
|
|
|
Middle East and Africa Capillary Blood Collection and Sampling Devices Treatment Market Analysis and Size
The capillary blood collection and sampling devices treatment market is being driven by the increasing number of hospital surgical procedures. Blood collection products help patients undergoing chemotherapy, dialysis, organ and tissue transplantation, and complex surgeries. More people are getting regular health checks because of the rising prevalence of chronic and lifestyle diseases caused by sedentary lifestyles. As a result, the demand for capillary blood collection and sampling devices as the primary method of disease diagnosis has increased.
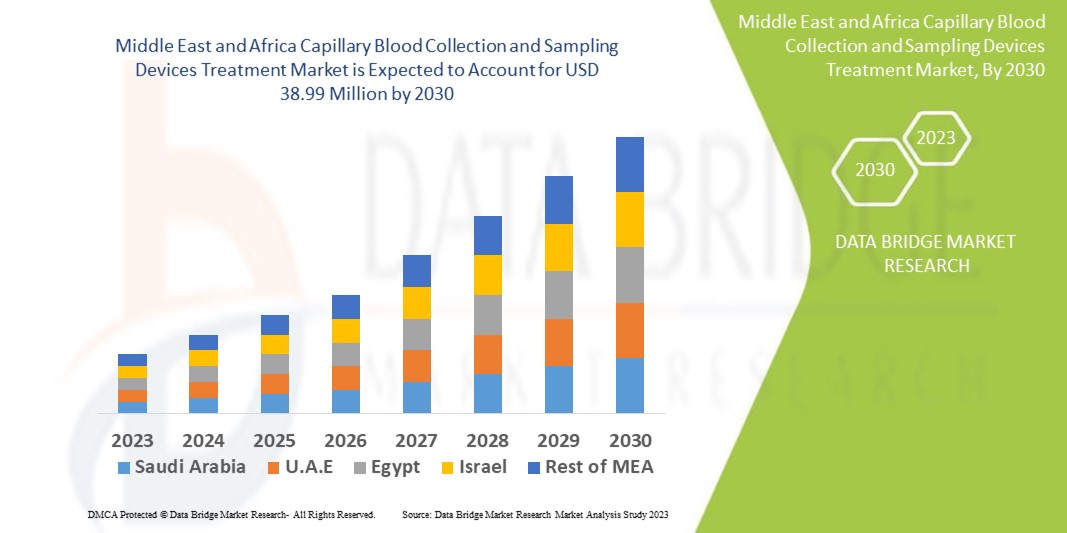
Data Bridge Market Research analyses that the capillary blood collection and sampling devices treatment market, which is USD 28.49 million in 2022, is expected to reach USD 38.99 million by 2030, at a CAGR of 4.0% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Middle East and Africa Capillary Blood Collection and Sampling Devices Treatment Market Scope and Segmentation
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 - 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, Pricing in USD |
|
Segments Covered |
Product (Blood Sampling Devices, Capillary Blood Collection Devices, Rapid Test Cassette, Remote Capillary Blood Collection Device, Wearable Capillary Blood Collection Device), Modality (Manual Sampling, Automated/Autoinjection Sampling), Mode of Administration (Puncture, Incision), Application (Cardiovascular Disease, Infection and Infectious Disease, Respiratory Diseases, Cancers, Rheumatoid Arthritis, Others), Platform (Enzyme Immunoassay Platform (ELISA Platform), PCR Platform, Lateral Flow Immunoassay Platform, ELTABA Platform, Others), Procedure (Conventional, Point of Care Testing), Age Group (Geriatrics, Infant, Pediatric, Adult), Test Type (Whole Blood Test, Dried Blood Spot Tests, Plasma/Serum Protein Tests, Liver Panel/Liver Profile/Liver Function Tests, Comprehensive Metabolic Panel (CMP) Tests, Others), Technology (Volumetric Absorptive Microsampling, Capillary Electrophoresis-Based Chemical Analysis, Others), Material (Plastic, Glass, Stainless Steel, Ceramic), End User (Laboratories, Home Care Setting), Distribution Channel (Direct Tender, Retail Sales, Others) |
|
Countries Covered |
Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA) |
|
Market Players Covered |
B.D. (U.S.), Terumo BCT Inc. (U.S.), Fresenius KABI AG (Germany), Grifols S.A. (Spain), Nipro Medical Corporation (U.S.), Greiner AG (Austria), Quest Diagnostics Incorporated (U.S.), Mitra Industries Private Limited (India), SARSTEDT AG & Co. K.G. (Germany), CML Biotech (India), Macopharma (France), Neomedic Limited (U.S.), Haemonetics Corporation (U.S.), ICU Medical, Inc. (U.S.), SB-KAWASUMI LABORATORIES, INC. (Japan), Retractable Technologies Inc. (U.S.) |
|
Market Opportunities |
|
Market Definition
Capillary blood collection devices are used to extract capillary blood. Capillary blood can be obtained through a finger, earlobe, or heel puncture. It is also possible to make an incision in the skin. Capillary blood collection and sampling techniques have gained popularity because they extract a precise amount of blood while lowering the risk of anaemia. For collecting capillary blood, the volumetric microsampling technique is now widely used. Furthermore, new technological advancements are being made to meet the needs of doctors and patients.
Middle East and Africa Capillary Blood Collection and Sampling Devices Treatment Market Dynamics
Drivers
- Increase in demand for safe blood collection technologies
Clinical diagnostics are important in many medical fields and in healthcare delivery. Blood sample collection is critical to laboratory activities because millions of blood samples are collected each year for chemical and haematological testing to diagnose diseases. The risky practise of blood collection, also known as phlebotomy, can have serious consequences for patients, including fainting, pain at the puncture site, nerve damage, and hematoma. Due to a shortage of skilled professionals, poor venipuncture practise has resulted in anatomical structures near the point of needle entry being injured.
Furthermore, capillary blood testing requires less blood volume and is quick and simple to perform. Physicians and patients prefer capillary blood sampling due to the advantages it has over venous blood sampling. As a result, there is a greater demand for more efficient capillary collection devices, which serves as a market driver.
- The rising availability of POC diagnostics
Point-of-care testing is diagnostic testing performed at the bedside of patients in their homes. It can also be done at any time and from any location. As a result, it is also known as bedside testing. Previously, testing was limited to the laboratories to which the specimen was sent, and obtaining the sample could take anywhere from hours to days. It not only saves time, but it also enables clinicians to provide quality and effective patient care in the patient's home. Because of its advantages, point-of-care diagnostics is becoming more popular.
According to various reports, the total value of the capillary blood collection and sampling devices treatment market was USD 40-45 billion, with POC diagnostics contributing USD 12-13 billion.
The demand for POC testing has increased as health awareness, and the incidence of infectious diseases have increased. Traditional arterial and venous blood collection methods are painful and invasive, with a higher risk of needlestick injuries and contamination if not performed by trained personnel. On the other hand, the capillary blood collection method is less invasive, causes less pain, and has few side effects. Furthermore, it does not necessitate the use of skilled professionals; thus, it is a point care testing technique. Capillary collection devices have advanced in tandem with the rise in POC testing.
Opportunities
- Product innovation and technological advancements in capillary blood collection devices
Blood collection is an important step in the delivery of healthcare. 70% to 80% of clinical decisions are made based on blood sample analysis. Many companies are using safer, easier, and more efficient methods to collect blood samples, thanks to the growing trend of blood collection innovation. For pharmacy clinics, capillary blood sampling is an excellent technique. This method is preferred over venipuncture due to its ease of use and safety; it also eliminates the need for skilled technicians. One disadvantage of finger sticks is that the blood droplets vary in size. This problem can be solved by introducing new and innovative products.
For instance- TAP, the world's first device that draws blood with a push button, was introduced by Seventh Sense Biosystems. This device is worn on the upper arm and draws blood with the push of a button. This makes high-quality lab testing more convenient and cost-effective.
Restraints/Challenges
- Higher risk related to the capillary blood assortment technologies
Higher risks associated with capillary blood collection technologies and the disadvantages of micro-collection of blood are expected to limit market growth during the forecast period. Capillary blood collection can occasionally cause blood cells to rupture, resulting in inaccurate results. Both methods of collection can cause bleeding and infection.
This capillary blood collection and sampling devices treatment market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the capillary blood collection and sampling devices treatment market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
COVID-19 Impact on the Capillary Blood Collection and Sampling Devices Treatment Market
The COVID-19 pandemic has created both challenges and opportunities in the global market. Due to global travel and trade restrictions, supply chain disruptions of medical devices/equipment and essential medical supplies were common during the pandemic. The virus outbreak, on the other hand, is driving significant growth in the point-of-care (POC) testing devices segment. As the virus spread, the number of infected patients increased rapidly, necessitating increased blood collection via various safety measures for antibody testing.
Recent developments
- In March 2020, B.D. and BioMedomics, a privately held clinical diagnostics company based in North Carolina, announced the development of a 15-minute at-home rapid test to assess current COVID-19 exposure. This is a novel testing device that will increase revenue generation.
Middle East and Africa Capillary Blood Collection and Sampling Devices Treatment Market Scope
The capillary blood collection and sampling devices treatment market is segmented on the basis of product, modality, mode of administration, application, platform, procedure, age group, test type, technology, material, end user, distribution channel. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product
- Blood Sampling Devices
- Capillary Blood Collection Devices
- Rapid Test Cassette
- Remote Capillary Blood Collection Device
- Wearable Capillary Blood Collection Device
Modality
- Manual Sampling
- Automated/Autoinjection Sampling
Mode of Administration
- Puncture
- Incision
Application
- Cardiovascular Disease
- Infection and Infectious Disease
- Respiratory Diseases
- Cancers
- Rheumatoid Arthritis
- Others
Platform
- Enzyme Immunoassay Platform (Elisa Platform)
- PCR Platform
- Lateral Flow Immunoassay Platform
- ELTABA Platform
- Others
Procedure
- Conventional
- Point of Care Testing
Age Group
- Geriatrics
- Infant
- Pediatric
- Adult
Test Type
- Whole Blood Test
- Dried Blood Spot Tests
- Plasma/Serum Protein Tests
- Liver Panel/Liver Profile/Liver Function Tests
- Comprehensive Metabolic Panel (CMP) Tests
- Others
Technology
- Volumetric Absorptive Microsampling
- Capillary Electrophoresis-Based Chemical Analysis
- Others
Material
- Plastic
- Glass
- Stainless Steel
- Ceramic
End User
- Laboratories
- Home Care Setting
Distribution Channel
- Direct Tender
- Retail Sales
- Others
Capillary Blood Collection and Sampling Devices Treatment Market Regional Analysis/Insights
The capillary blood collection and sampling devices treatment market is analyzed and market size insights and trends are provided by country, product, modality, mode of administration, application, platform, procedure, age group, test type, technology, material, end user, distribution channel as referenced above.
The countries covered in the capillary blood collection and sampling devices treatment market report are Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA).
South Africa has control over the Middle East and Africa region due to increased adoption of advanced technology. The Middle East and Africa capillary blood collection and sampling devices market is expanding due to rising health-care costs.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Healthcare Infrastructure Growth Installed base and New Technology Penetration
The capillary blood collection and sampling devices treatment market also provides you with detailed market analysis for every country growth in healthcare expenditure for capital equipment, installed base of different kind of products for capillary blood collection and sampling devices treatment market, impact of technology using life line curves and changes in healthcare regulatory scenarios and their impact on the capillary blood collection and sampling devices treatment market. The data is available for historic period 2011-2021.
Competitive Landscape and Capillary Blood Collection and Sampling Devices Treatment Market Share Analysis
The capillary blood collection and sampling devices treatment market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to capillary blood collection and sampling devices treatment market.
Some of the major players operating in the capillary blood collection and sampling devices treatment market are:
- B.D. (U.S.)
- Terumo BCT Inc. (U.S.)
- Fresenius KABI AG (Germany)
- Grifols S.A. (Spain)
- Nipro Medical Corporation (U.S.)
- Greiner AG (Austria)
- Quest Diagnostics Incorporated (U.S.)
- Mitra Industries Private Limited (India)
- SARSTEDT AG & Co. KG (Germany)
- CML Biotech (India)
- Macopharma (France)
- Neomedic Limited (U.S.)
- Haemonetics Corporation (U.S.)
- ICU Medical, Inc. (U.S.)
- SB-KAWASUMI LABORATORIES, INC. (Japan)
- Retractable Technologies Inc. (U.S.)
SKU-
Get online access to the report on the World's First Market Intelligence Cloud
- Interactive Data Analysis Dashboard
- Company Analysis Dashboard for high growth potential opportunities
- Research Analyst Access for customization & queries
- Competitor Analysis with Interactive dashboard
- Latest News, Updates & Trend analysis
- Harness the Power of Benchmark Analysis for Comprehensive Competitor Tracking
Table of Content
1. INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF MIDDLE EAST AND AFRICA CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2. MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 ARRIVING AT THE MIDDLE EAST AND AFRICA CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES TREATMENT MARKET
2.2.1 VENDOR POSITIONING GRID
2.2.2 TECHNOLOGY LIFE LINE CURVE
2.2.3 MARKET GUIDE
2.2.4 COMPANY POSITIONING GRID
2.2.5 COMAPANY MARKET SHARE ANALYSIS
2.2.6 MULTIVARIATE MODELLING
2.2.7 TOP TO BOTTOM ANALYSIS
2.2.8 STANDARDS OF MEASUREMENT
2.2.9 VENDOR SHARE ANALYSIS
2.2.10 SALES VOULME
2.2.11 DATA POINTS FROM KEY PRIMARY INTERVIEWS
2.2.12 DATA POINTS FROM KEY SECONDARY DATABASES
2.3 MIDDLE EAST AND AFRICA CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES TREATMENT MARKET: RESEARCH SNAPSHOT
2.4 ASSUMPTIONS
3. MARKET OVERVIEW
3.1 DRIVERS
3.2 RESTRAINTS
3.3 OPPORTUNITIES
3.4 CHALLENGES
4. EXECUTIVE SUMMARY
5. PREMIUM INSIGHTS
5.1 PESTLE ANALYSIS
5.2 PORTER’S ANALYSIS
5.3 PATENT ANALYSIS
5.4 TECHNOLOGICAL ADVANCEMENTS IN DIAGNOSTICS
5.5 INTRA-ABDOMINAL PRESSURE MEASUREMENT DEVICES MARKET ANALYSIS (QUALITATIVE AND QUANTITATIVE ANALYSIS)
6. INDUSTRY INSIGHTS
6.1 KEY PRICING STRATEGIES
6.2 INTERVIEWS WITH DIAGNOSTIC COMPANIES
6.3 INTERVIEWS WITH PHYSICIANS
6.4 INTERVIEWS WITH RESEARCH SCHOLARS
6.5 OTHER KOL SNAPSHOTS
7. REGULATORY FRAMEWORK
8. MIDDLE EAST AND AFRICA CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES TREATMENT MARKET, BY PRODUCT
8.1 OVERVIEW
8.2 CAPILLARY BLOOD COLLECTION DEVICES
8.2.1 LANCETS
8.2.1.1. BY TYPE
8.2.1.1.1. SAFETY LANCETS
8.2.1.1.2. LASER LANCET
8.2.1.1.3. HEEL PUNCTURE LANCETS
8.2.1.1.4. CONTACT-ACTIVATED LANCET
8.2.1.1.5. HOMECARE LANCETS
8.2.1.1.6. OTHERS
8.2.1.2. BY FLOW
8.2.1.2.1. LOW FLOW
8.2.1.2.2. MEDIUM FLOW
8.2.1.2.3. HIGH FLOW
8.2.2 MICRO-CONTAINER TUBES
8.2.2.1. BY MATERIAL
8.2.2.1.1. PLASTIC
8.2.2.1.2. GLASS
8.2.2.1.3. STAINLESS STEEL
8.2.2.1.4. OTHERS
8.2.2.2. BY TYPE
8.2.2.2.1. SERUM TUBES
8.2.2.2.2. SERUM-SEPARATING TUBES
8.2.2.2.3. RAPID SERUM TUBES
8.2.2.2.4. EDTA TUBES
8.2.2.2.5. COAGULATION TUBES
8.2.2.2.6. HEPARIN TUBES
8.2.2.2.7. PLASMA SEPARATOR TUBE
8.2.2.2.8. ERYTHROCYTE SEDIMENTATION RATE (ESR) TUBES
8.2.2.2.9. OTHERS
8.2.3 MICRO-HEMATOCRIT TUBES
8.2.3.1. BY TYPE
8.2.3.1.1. HEPARINIZED
8.2.3.1.2. NON- HEPARINIZED
8.2.3.2. BY MATERIAL
8.2.3.2.1. PLASTIC
8.2.3.2.2. GLASS
8.2.3.2.3. STAINLESS STEEL
8.2.3.2.4. OTHERS
8.2.4 WARMING DEVICES
8.2.5 BLOOD BAGS
8.2.5.1. SINGLE BLOOD BAG
8.2.5.2. DOUBLE BLOOD BAG
8.2.5.3. TRIPLE BLOOD BAG
8.2.5.4. OTHERS
8.2.6 TUBE HOLDER
8.2.7 OTHERS
8.3 BLOOD SAMPLING DEVICES
8.3.1 NEEDLES AND SYRINGES
8.3.2 NEEDLE PROTECTION DEVICE
8.3.3 BLOOD TRANSFER UNIT
8.3.4 TUBE HOLDER
8.3.5 HAEMATOCRIT READER
8.3.6 VIALS
8.3.7 OTHERS
8.4 AUTO-INJECTION BASED DEVICES
8.4.1 PEDIATRIC
8.4.2 ADULT BASED
8.5 REMOTE CAPILLARY BLOOD COLLECTION DEVICE
8.5.1 BY PRODUCT
8.5.2 COLLECTION KITS
8.5.2.1. 10 µL
8.5.2.2. 20 µL
8.5.2.3. 30 µL
8.5.3 CARTRIDGE
8.5.4 CLAMSHELL
8.5.5 AUTORACK
8.6 WEARABLE CAPILLARY BLOOD COLLECTION DEVICE
8.6.1 NEEDLE FREE
8.6.2 MICRONEEDLE
8.7 OTHERS
9. MIDDLE EAST AND AFRICA CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES TREATMENT MARKET, BY MODALITY
9.1 OVERVIEW
9.2 MANUAL DEVICES
9.3 AUTOMATED DEVICES
10. MIDDLE EAST AND AFRICA CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES TREATMENT MARKET, BY MATERIAL
10.1 OVERVIEW
10.2 PLASTIC
10.3 GLASS
10.4 STAINLESS STEEL
10.5 CERAMIC
10.6 OTHERS
11. MIDDLE EAST AND AFRICA CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES TREATMENT MARKET, BY PUNCTURE TYPE
11.1 OVERVIEW
11.2 INCISION
11.3 PUNCTURE
11.3.1 FINGER PUNCTURE
11.3.2 HEEL PUNCTURE
12. MIDDLE EAST AND AFRICA CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES TREATMENT MARKET, BY PROCEDURE
12.1 OVERVIEW
12.2 CONVENTIONAL
12.3 POINT OF CARE TESTING
13. MIDDLE EAST AND AFRICA CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES TREATMENT MARKET, BY AGE GROUP
13.1 OVERVIEW
13.2 INFANT
13.3 PEDIATRIC
13.4 ADULT
13.5 GERIATRICS
14. MIDDLE EAST AND AFRICA CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES TREATMENT MARKET, BY TEST TYPE
14.1 OVERVIEW
14.2 WHOLE BLOOD TEST
14.2.1 CAPILLARY BLOOD COLLECTION DEVICES
14.2.2 BLOOD SAMPLING DEVICES
14.2.3 AUTO-INJECTION BASED DEVICES
14.2.4 REMOTE CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICE
14.2.5 WEARABLE CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICE
14.2.6 SELF-ADMINISTERED BLOOD COLLECTOR DEVICE
14.2.7 OTHERS
14.3 PLASMA/ SERUM PROTEIN TESTS
14.3.1 CAPILLARY BLOOD COLLECTION DEVICES
14.3.2 BLOOD SAMPLING DEVICES
14.3.3 AUTO-INJECTION BASED DEVICES
14.3.4 REMOTE CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICE
14.3.5 WEARABLE CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICE
14.3.6 SELF-ADMINISTERED BLOOD COLLECTOR DEVICE
14.3.7 OTHERS
14.4 COMPREHENSIVE METABOLIC PANEL (CMP) TESTS
14.4.1 CAPILLARY BLOOD COLLECTION DEVICES
14.4.2 BLOOD SAMPLING DEVICES
14.4.3 AUTO-INJECTION BASED DEVICES
14.4.4 REMOTE CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICE
14.4.5 WEARABLE CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICE
14.4.6 SELF-ADMINISTERED BLOOD COLLECTOR DEVICE
14.4.7 OTHERS
14.5 LIVER PANEL / LIVER PROFILE/ LIVER FUNCTION TESTS
14.5.1 CAPILLARY BLOOD COLLECTION DEVICES
14.5.2 BLOOD SAMPLING DEVICES
14.5.3 AUTO-INJECTION BASED DEVICES
14.5.4 REMOTE CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICE
14.5.5 WEARABLE CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICE
14.5.6 SELF-ADMINISTERED BLOOD COLLECTOR DEVICE
14.5.7 OTHERS
14.6 DRIED BLOOD SPOT TESTS
14.6.1 CAPILLARY BLOOD COLLECTION DEVICES
14.6.2 BLOOD SAMPLING DEVICES
14.6.3 AUTO-INJECTION BASED DEVICES
14.6.4 REMOTE CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICE
14.6.5 WEARABLE CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICE
14.6.6 SELF-ADMINISTERED BLOOD COLLECTOR DEVICE
14.6.7 OTHERS
14.7 OTHERS
15. MIDDLE EAST AND AFRICA CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES TREATMENT MARKET, BY APPLICATION
15.1 OVERVIEW
15.2 DIAGNOSTIC
15.2.1 INFECTIOUS DISEASES
15.2.1.1. CAPILLARY BLOOD COLLECTION DEVICES
15.2.1.2. BLOOD SAMPLING DEVICES
15.2.1.3. AUTO-INJECTION BASED DEVICES
15.2.1.4. REMOTE CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES
15.2.1.5. WEARABLE CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICE
15.2.1.6. SELF-ADMINISTERED BLOOD COLLECTOR DEVICE
15.2.1.7. OTHERS
15.2.2 NON-INFECTIOUS DISEASES
15.2.2.1. CAPILLARY BLOOD COLLECTION DEVICES
15.2.2.2. BLOOD SAMPLING DEVICES
15.2.2.3. AUTO-INJECTION BASED DEVICES
15.2.2.4. REMOTE CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES
15.2.2.5. WEARABLE CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES
15.2.2.6. SELF-ADMINISTERED BLOOD COLLECTOR DEVICE
15.2.2.7. OTHERS
15.2.3 OTHERS
15.3 TREATMENT
15.3.1 CAPILLARY BLOOD COLLECTION DEVICES
15.3.2 BLOOD SAMPLING DEVICES
15.3.3 AUTO-INJECTION BASED DEVICES
15.3.4 REMOTE CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES
15.3.5 WEARABLE CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICE
15.3.6 SELF-ADMINISTERED BLOOD COLLECTOR DEVICE
15.3.7 OTHERS
16. MIDDLE EAST AND AFRICA CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES TREATMENT MARKET, BY TECHNOLOGY
16.1 OVERVIEW
16.2 VOLUMETRIC ABSORPTIVE MICROSAMPLING
16.3 VENIPUNCTURE
16.4 CAPILLARY ELECTROPHORESIS-BASED CHEMICAL ANALYSIS
16.5 OTHERS
17. MIDDLE EAST AND AFRICA CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES TREATMENT MARKET, BY END USER
17.1 OVERVIEW
17.2 HOSPITALS
17.3 CLINICS
17.4 PATHOLOGY LABORATORIES
17.5 BLOOD BANKS
17.6 RESEARCH & ACADEMIC LABORATORIES
17.7 HOME CARE SETTING
17.8 OTHERS
18. MIDDLE EAST AND AFRICA CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES TREATMENT MARKET, BY END USER
18.1 OVERVIEW
18.2 DIRECT TENDER
18.3 RETAIL SALES
18.4 OTHERS
19. MIDDLE EAST AND AFRICA CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES TREATMENT MARKET, BY GEOGRAPHY
MIDDLE EAST AND AFRICA CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES TREATMENT MARKET, (ALL SEGMENTATION PROVIDED ABOVE IS REPRESENTED IN THIS CHAPTER BY COUNTRY)
19.1 SOUTH AFRICA
19.2 ISRAEL
19.3 SAUDI ARABIA
19.4 UAE
19.5 REST OF MIDDLE EAST AND AMERICA
20. MIDDLE EAST AND AFRICA CAPILLARY BLOOD COLLECTION AND SAMPLING DEVICES TREATMENT MARKET, COMPANY LANDSCAPE
20.1 COMPANY SHARE ANALYSIS: MIDDLE EAST AND AFRICA
20.2 MERGERS & ACQUISITIONS
20.3 NEW PRODUCT DEVELOPMENT & APPROVALS
20.4 EXPANSIONS & PARTNERSHIP
20.5 REGULATORY CHANGES
21. COMPANY PROFILE
21.1 BD
21.1.1 COMPANY OVERVIEW
21.1.2 REVENUE ANALYSIS
21.1.3 GEOGRAPHIC PRESENCE
21.1.4 PRODUCT PORTFOLIO
21.1.5 RECENT DEVELOPMENTS
21.2 IMPROVE MEDICAL
21.2.1 COMPANY OVERVIEW
21.2.2 REVENUE ANALYSIS
21.2.3 GEOGRAPHIC PRESENCE
21.2.4 PRODUCT PORTFOLIO
21.2.5 RECENT DEVELOPMENTS
21.3 HEBEI XINLE SCI&TECH CO., LTD.
21.3.1 COMPANY OVERVIEW
21.3.2 GEOGRAPHIC PRESENCE
21.3.3 PRODUCT PORTFOLIO
21.3.4 RECENT DEVELOPMENTS
21.4 TERUMO MEDICAL CORPORATION
21.4.1 COMPANY OVERVIEW
21.4.2 REVENUE ANALYSIS
21.4.3 GEOGRAPHIC PRESENCE
21.4.4 PRODUCT PORTFOLIO
21.4.5 RECENT DEVELOPMENTS
21.5 SARSTEDT AG & CO. KG
21.5.1 COMPANY OVERVIEW
21.5.2 REVENUE ANALYSIS
21.5.3 GEOGRAPHIC PRESENCE
21.5.4 PRODUCT PORTFOLIO
21.5.5 RECENT DEVELOPMENTS
21.6 GREINER BIO-ONE PREANALYTICS
21.6.1 COMPANY OVERVIEW
21.6.2 GEOGRAPHIC PRESENCE
21.6.3 REVENUE ANALYSIS
21.6.4 PRODUCT PORTFOLIO
21.6.5 RECENT DEVELOPMENTS
21.7 BIO RAD LABORATORIES
21.7.1 COMPANY OVERVIEW
21.7.2 GEOGRAPHIC PRESENCE
21.7.3 PRODUCT PORTFOLIO
21.7.4 RECENT DEVELOPMENTS
21.8 INTERVAC TECHNOLOGY
21.8.1 COMPANY OVERVIEW
21.8.2 GEOGRAPHIC PRESENCE
21.8.3 PRODUCT PORTFOLIO
21.8.4 RECENT DEVELOPMENTS
21.9 TRAJAN SCIENTIFIC AND MEDICAL
21.9.1 COMPANY OVERVIEW
21.9.2 REVENUE ANALYSIS
21.9.3 GEOGRAPHIC PRESENCE
21.9.4 PRODUCT PORTFOLIO
21.9.5 RECENT DEVELOPMENTS
21.10 F. HOFFMANN-LA ROCHE AG
21.10.1 COMPANY OVERVIEW
21.10.2 GEOGRAPHIC PRESENCE
21.10.3 PRODUCT PORTFOLIO
21.10.4 RECENT DEVELOPMENTS
21.11 ICU MEDICAL
21.11.1 COMPANY OVERVIEW
21.11.2 GEOGRAPHIC PRESENCE
21.11.3 PRODUCT PORTFOLIO
21.11.4 RECENT DEVELOPMENTS
21.12 DANAHER CORPORATION
21.12.1 COMPANY OVERVIEW
21.12.2 REVENUE ANALYSIS
21.12.3 GEOGRAPHIC PRESENCE
21.12.4 PRODUCT PORTFOLIO
21.12.5 RECENT DEVELOPMENTS
21.13 LASEC GROUP
21.13.1 COMPANY OVERVIEW
21.13.2 REVENUE ANALYSIS
21.13.3 GEOGRAPHIC PRESENCE
21.13.4 PRODUCT PORTFOLIO
21.13.5 RECENT DEVELOPMENTS
21.14 MEDTRONIC
21.14.1 COMPANY OVERVIEW
21.14.2 REVENUE ANALYSIS
21.14.3 GEOGRAPHIC PRESENCE
21.14.4 PRODUCT PORTFOLIO
21.14.5 RECENT DEVELOPMENTS
21.15 ASP GLOBAL
21.15.1 COMPANY OVERVIEW
21.15.2 REVENUE ANALYSIS
21.15.3 GEOGRAPHIC PRESENCE
21.15.4 PRODUCT PORTFOLIO
21.15.5 RECENT DEVELOPMENTS
21.16 FRESENIUS KABI
21.16.1 COMPANY OVERVIEW
21.16.2 REVENUE ANALYSIS
21.16.3 GEOGRAPHIC PRESENCE
21.16.4 PRODUCT PORTFOLIO
21.16.5 RECENT DEVELOPMENTS
21.17 ABBOTT
21.17.1 COMPANY OVERVIEW
21.17.2 GEOGRAPHIC PRESENCE
21.17.3 PRODUCT PORTFOLIO
21.17.4 RECENT DEVELOPMENTS
21.18 NIPRO MEDICAL CORPORATION
21.18.1 COMPANY OVERVIEW
21.18.2 REVENUE ANALYSIS
21.18.3 GEOGRAPHIC PRESENCE
21.18.4 PRODUCT PORTFOLIO
21.18.5 RECENT DEVELOPMENTS
22. RELATED REPORTS
23. CONCLUSION
24. QUESTIONNAIRE
25. ABOUT DATA BRIDGE MARKET RESEARCH

Research Methodology
Data collection and base year analysis are done using data collection modules with large sample sizes. The stage includes obtaining market information or related data through various sources and strategies. It includes examining and planning all the data acquired from the past in advance. It likewise envelops the examination of information inconsistencies seen across different information sources. The market data is analysed and estimated using market statistical and coherent models. Also, market share analysis and key trend analysis are the major success factors in the market report. To know more, please request an analyst call or drop down your inquiry.
The key research methodology used by DBMR research team is data triangulation which involves data mining, analysis of the impact of data variables on the market and primary (industry expert) validation. Data models include Vendor Positioning Grid, Market Time Line Analysis, Market Overview and Guide, Company Positioning Grid, Patent Analysis, Pricing Analysis, Company Market Share Analysis, Standards of Measurement, Global versus Regional and Vendor Share Analysis. To know more about the research methodology, drop in an inquiry to speak to our industry experts.
Customization Available
Data Bridge Market Research is a leader in advanced formative research. We take pride in servicing our existing and new customers with data and analysis that match and suits their goal. The report can be customized to include price trend analysis of target brands understanding the market for additional countries (ask for the list of countries), clinical trial results data, literature review, refurbished market and product base analysis. Market analysis of target competitors can be analyzed from technology-based analysis to market portfolio strategies. We can add as many competitors that you require data about in the format and data style you are looking for. Our team of analysts can also provide you data in crude raw excel files pivot tables (Fact book) or can assist you in creating presentations from the data sets available in the report.